Document Type : Original Research Article
Authors
Department of Chemistry, School of Sciences, Payame Noor University (PNU), 19395-4697, Tehran, Iran
Abstract
2-amino-4H-benzopyran derivatives have received considerable attention due to their valuable biological and medicinal properties such as anticoagulant, antispasmodic, diuretic, and anti-cancer activities. Benzopyran derivatives have strong relaxing activity on blood vessels, cardiac muscles, and smooth muscles. The present study reports the easy synthesis of 4H-benzopyran derivatives by using aldehyde, malononitrile, and dimedone in the presence of cerium(IV) oxide nanopowder as a non-toxic catalyst. The advantages of this method include catalyst recovery, high efficiency, short reaction time, and ease of operation method.
Graphical Abstract
Keywords
Main Subjects
Introduction
According to its definition, a multi-component reaction occurs among three or more reactants together in one step, and the final product, which certainly contains the atoms of raw materials, is synthesized. Multi-component reactions are a very important tool for the synthesis of various complex organic molecules from simple structural components through forming carbon-carbon and carbon-heteroatom bonds within a container [1, 2]. The advantages of this reaction include ease of reaction, high efficiency, shorter reaction time, structural variability, low cost, and less by-products [3].
Green chemistry scientists seek to replace the recent processes with healthier chemical processes or to provide healthier products to society by substituting healthier raw materials or performing reactions under safer conditions. Some of them try to bring chemistry closer to biochemistry because biochemical reactions have taken place over millions of years and have not posed alarming challenges for either humans or the environment. Many of these reactions occur naturally and do not require high temperatures and pressures. Their products also return simply to the material cycle, and their by-products are useful to living things. The design of chemical reactions and processes has created countless new opportunities for chemists, and any chemist can improve, reduce costs, and increase the efficiency of any known reaction that has taken place in factories and university laboratories for years. Therefore, it seems that the opportunities provided for chemists during the ancient history of this science are also provided for today's chemists to modify what they have left in the history of chemistry to leave healthier memories for the future.
Pyran derivatives have a wide range of medicinal and biological applications such as anti-coagulant, anti-cancer, anti-depressant, anti-tumor, and diuretic properties [4]. These compounds are used in the treatment of many diseases such as Alzheimer's, Huntington's, Parkinson's, AIDS, and Down syndrome [5]. Coumarin naturally exists in nature and many of its derivatives are known to have anticoagulant activity. For example, Warfarin, as a rodenticide toxin, is a useful anticoagulant (Scheme 1a) [6]. Flavones that contain a pyran ring are pigments that color a variety of citrus fruits, vegetables, and tea. These compounds have antioxidant properties (Scheme 1b) [7]. The compound Nedocromil sodium is an anti-allergic agent (Scheme 1c) [7]. Lacton 2H-pyran-2-one is used as an antibacterial and antifungal in fruits (Scheme 1d) [8]. The compounds 2-amino-4H-pyran (Scheme 1e) and the 2-amino-5-oxo-5,6,6,7-tetrahydrobenzo [b] pyran have antibacterial properties (Scheme 1f) [9].
Following the discovery of the medicinal properties of these derivatives in recent years and by using nano catalyst for the synthesis of heterocyclic compounds [10⎼16], we evaluate the synthesis of these compounds by using nano-CeO2 as an efficient catalyst.
Experimental
Materials and methods
The 9200-Barnstead Electrothermal device was used to measure the melting point of the products. The progress of the reaction was assessed with the TLC technique by using UV lamps at two wavelengths of 254 and 356 nm. IR spectra were recorded by using the FT-IR Tensor 27 via KBr tablets. 1H NMR spectra were obtained by using Bruker DPX 400MHz spectrometer.
Solvents and chemicals were purchased from Merck. The structure of the obtained products was compared with the spectra and physical data recorded in the references.
Preparation of cerium(IV) oxide nanopowder
Cerium(IV) oxide nanopowder was prepared by precipitation method by using CeCl3.7H2O (Merck, purity >99.5%) and NH3 (Merck, purity >99%). First, CeCl3.7H2O was dissolved in deionized water, and then the mixture was stirred for 30 minutes. After that NH3 (0.5 mol) was added to the aqueous solution until a jelly form was made at a pH of about 8.5. The resulting synthetic gel was then washed with boiling distilled water and dried at 80 °C for 24 hours. The gel was dried and calcined at 700 °C for two hours. The SEM and TEM spectra of the catalyst are as follows (Figures 1 and 2) [17].
Synthesis of 2-amino-4H-benzopyran derivatives in the presence of nano cerium oxide
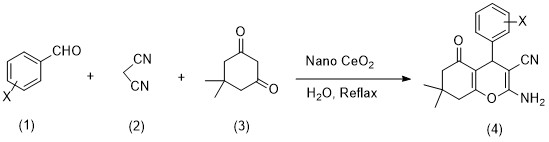
In a 25 mL flask, aryl aldehyde (1 mmol), dimedone (1 mmol), malononitrile (1 mmol), catalyst (0.05 g), and water (5 cc) were stirred under reflux conditions. The progress of the reaction was tracked by using TLC in a mixture of ethyl acetate and n-hexane in 1:2 ratio. After the reaction completion and cooling at room temperature, the mixture was filtered and recrystallized in ethanol (Scheme 1). Melting point, as well as FT-IR and 1HNMR spectra of the obtained crystals were measured and compared with the references.
2-amino-5,6,7,8-tetrahydro-5-oxo-4-phenyl-7,7-dimethyl-4H-Benzo-[b]-pyran-3-carbonitrile (4a)
IR (KBr) (νmax/ cm-1): 3393, 3295, 2938, 2203, 1688, and 1607. 1H-NMR (CDCl3, 500 MHz): δ 0.99 (3H, singlet), 1.07 (3H, singlet), 2.4 (1H, AB quartet of doublets), 2.28 (1H, AB quartet of doublets), 2.54 (2H, broad singlet), 3.39 (singlet, NH2), 4.34 (1H, s), 7.12 (3H, multiplet), 7.6 (2H, multiplet).
Results and Discussion
Nanocatalysts play a key role in chemistry. As the size of the catalysts decreases, their efficiency increases. Nanocatalysis science is a part of nanochemistry that synthesizes nano-scale catalysts. By the nanometric properties of catalysts, their activity, selectivity, and stability can be controlled.
Scheme 1. Coumarin compounds with medicinal properties

Figure 1. SEM spectra of nano CeO2
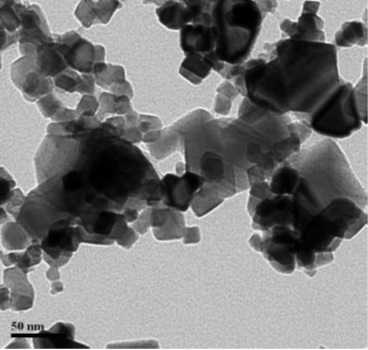
Figure 2. TEM spectra of nano CeO2
With the introduction of nanotechnology into the catalyst industry, nanocatalysts entered the industry and laboratories. The most important achievement of nanotechnology in catalyst science is the uniformity of size and distribution of active catalytic pores, which prevents different unwanted reactions and by-products. Moreover, the high costs of designing and manufacturing separation units will be eliminated and the environmental problems arising from by-products will be solved. Some characteristics of nanocatalysts include high selectivity and efficiency, high tendency to agglomerate, high diversity, chemical modification capability, ability to recover from the reaction mixture, and well-controlled size.
Cerium(IV) oxide, also known as (ceric oxide, ceria, and cerium dioxide), is a yellow powder with the chemical formula CeO2. Ceria powder can absorb very small amounts of carbon dioxide from the atmosphere. Researchers use ceria nanoparticles for various applications. These nanoparticles are used to coat metals, which help oxidation-reduction [18], and the study of catalytic converters of carbon monoxide and hydrocarbons. The recent studies have shown that ceria nanoparticles are useful for reducing free radical damage to rat brain cells, including neurons [19, 20]. The antioxidant effect of ceria nanoparticles increases the lifespan of neurons by reducing free radical damage in acute disorders [21]. Cerium oxide is used in crude oil refining as a catalyst in separation. This material in glass also allows for selective adsorption. Cerium(IV) oxide is used in glass production as a decolorizing compound. We used nano-cerium oxide as a catalyst for the synthesis of 2-amino-4H-benzopyran derivatives from aldehyde, dimedone, and malonotirile in water (Scheme 2).
Optimization of reaction conditions
First, the compression reaction of benzaldehyde, malononitrile, and dimedone was selected as the model reaction. Then, an attempt was made to obtain the best conditions for the model reaction. For this purpose, the optimal values of three important parameters (i.e. the amount of catalyst, solvent, and temperature) were investigated for this reaction.
Solvent selection
Initially, the reaction was performed without solvent, and the product was obtained with low efficiency in a long time. The reaction was then performed in water, ethanol, acetonitrile, and chloroform solvents. The best efficiency was water solvent in the shortest time (Table 1).
Optimization of reaction temperature
First, the reaction was performed at room temperature (25 °C) in water as the solvent for 240 minutes. This was a long time to react. Then, the reaction was performed at 25, 70, and 100 °C. The best efficiency was obtained in the shortest time at boiling water. The results are represented in Table 2.
Optimization of the amount of catalyst
For optimization, the reaction was performed with different amounts of cerium oxide nanocatalyst and it was found that the optimal amount for this catalyst was 0.05 g in the aqueous solvent (Table 3).
After optimizing the reaction conditions by using the model reaction, the synthesis of different 2-amino-4H-benzopyran derivatives with optimal conditions was investigated. As discussed in the previous sections, the optimal amount of catalyst was 0.05 g, the best solvent was water, and the best temperature was the boiling point. The results are indicated in Table 4.
Comparison of the performance of nano-CeO2 with a number of different catalysts in the synthesis of 2-amino-4H-benzopyran derivatives
To understand the catalytic merit of nano-CeO2, the results of this investigation were compared with those reported in literature with various catalysts (Table 5). Nano-CeO2 catalyst results in better or comparable yields under shorter reaction times.

Scheme 2. Synthesis of 2-amino-4Hbenzopyran derivatives
Table 1. Synthesis of 4a in the presence of different solvents by using nano-CeO2 as a catalyst
|
Entry |
Solvent |
Yield (%)a |
|
1 |
THF |
68 |
|
2 |
C2H5OH |
87 |
|
3 |
CH3CN |
85 |
|
4 |
CHCl3 |
71 |
|
5 |
Solvent-free |
87 |
|
6 |
Water |
92 |
aYields were analyzed by GC
Table 2. Comparison of various temperatures for the synthesis of 4a
| Entry | Time (h) | Temperature (°C) | Yield (%) |
|
1 |
3 |
25 |
66 |
|
2 |
3 |
70 |
75 |
|
3 |
3 |
reflux |
92 |
Table 3. Comparison of amount of catalysts for the synthesis of 4a
|
Entry |
Solvent |
Yield (%)a |
|
1 |
0.02 g |
80 |
|
2 |
0.03 g |
85 |
|
3 |
0.05 g |
92 |
|
4 |
0.08 g |
92 |
aYields were analyzed by GC
Table 4. Synthesis of substituted 2-amino-4H-Pyran catalyzed by nano-SnO2 complex

Table 5. Comparison of various catalysts for the synthesis of 2-amino-4H-Pyran derivatives
| Entry |
Catalyst |
Solvent |
Yield (%) |
Time |
Reference |
|
1 |
NaBr |
Solvet-free |
60-95 |
10-15 min |
[23] |
|
2 |
(S)-Proline |
H2O/EtOH |
78-98 |
30 min |
[24] |
|
3 |
HDMBAB |
H2O |
84-93 |
7-8 h |
[25] |
|
4 |
Na2SeO4 |
EtOH/H2O |
80-98 |
0.75-3 h |
[26] |
|
5 |
TMAH |
H2O |
79-93 |
0.5-2 h |
[27] |
|
6 |
TBAF |
H2O |
73-98 |
30-300 min |
[28] |
|
7 |
MgO |
EtOH/H2O |
90-96 |
22-33 min |
[29] |
|
8 |
Nano-SnO2 |
H2O |
89-97 |
8-20 min |
[30] |
|
9 |
Nano-CeO2 |
H2O |
90-98 |
5-15 min |
Present study |
Due to its empty d orbitals, cerium oxide nanocatalyst can accommodate pairs of electrons of the carbonyl group, and thus makes it more active. Therefore, the nucleophilic attack on the carbonyl is facilitated. In this reaction, the aromatic aldehyde is initially combined with malononitrile through the Knoevenagel condensation reaction and α-cyanonitrile derivative is obtained. After that, the active methylene reacts with C=C in α-cyanonitrile to produce a product that can be tautomerized. The nucleophilic attack of the OH group on the cyano (CN) leads to the production of an intermediate that is tautomerized to the target product (Scheme 3).

Scheme 3. Mechanism of 2-amino-4H-benzopyran
Conclusions
Pyrans and their derivatives have beneficial biological properties and various applications in the pharmaceutical and agricultural industries. Therefore, it has attracted the attention of many chemists. Since most of the reported synthetic methods are multi steps and have disadvantages such as high temperature, use of toxic solvents, long time, and low efficiency, in this study, we presented a useful and safe method to prepare the desired compounds. The most important advantages of this synthetic method are as follows: The reaction is multicomponent and one-pot, so there is no need to separate the intermediates, and thus it is an ideal method of synthesis. Use of water as the solvent instead of toxic and dangerous solvents such as dichloromethane. The reaction time is short time with high efficiency percentage. Use of smaller amounts of nanocatalysts and being environmentally friendly. Then, the advantages of this method include catalyst recovery, high efficiency, short reaction time, and easy operation method.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
How to cite this manuscript: Bita Baghernejad*, Seyyedeh Mojgan Hojati. Aqueous media preparation of 2-amino-4H-benzopyran derivatives using cerium oxide nanoparticles as a recyclable catalyst. Asian Journal of Green Chemistry, 6(3) 2022, 194-202. DOI: 10.22034/ajgc.2022.3.2