Document Type : Original Research Article
Authors
- Rathab Ali Ahmed 1
- Ola Hamad Salah 2
- Russul Reidh Abass 3
- Manal Morad Karim 4
- Shahad Abdulhadi Khuder 5
- Salam Ahjel 6
- Imad Ibrahim Dawood 7
1 Department of Medical Engineering, AL-Nisour University College, Baghdad, Iraq
2 Department of Medical Engineering Al-Manara College For Medical Sciences, Maysan, Iraq
3 Al-Farahidi University, College of Medical Technology, Medical Lab. Techniques Department, Baghdad, Iraq
4 College of Pharmacy, National University of Science and Technology, Dhi Qar, Iraq
5 Department of Medical Engineering, Al-Hadi University College, Baghdad, 10011, Iraq
6 Department of Pharmacy, Al-Zahrawi University College, Karbala, Iraq
7 Department of Medical Engineering, Mazaya University College, Nasiriyah, Dhi Qar, Iraq
Abstract
Heterogeneous photocatalysts have been widely utilized for the degradation of pharmaceuticals in wastewater. Under UV light irradiation, photocatalysis of the Sulfadiazine Hydrochloride (SFD) drug in wastewater using Pd/ZnO nanocomposite was studied. The nanocomposite was prepared using a hydrothermal process. The incorporation of Pd nanoparticles into the ZnO nanostructure increased the porosity and surface area, as well as the number of functional and active sites of the nanocomposite, which can improve the photocatalytic process of drug removal. According to structural analyses using TEM and SEM, Pd/ZnO refers to a highly stable and architectural morphology. The photocatalytic degradation process revealed that after 60 min, it led to the removal of the SFD drug, and a degradation efficiency of up to 85.77% was obtained using ZnO NPs, while the complete Pd/ZnO nanocomposite photocatalytic process was obtained after 60 min, with a degradation efficiency of up to 92.25%. The high effectiveness of the prepared surface of the Pd/ZnO nanocomposite on the degradation of SFD drugs from aqueous solutions was confirmed, and the results showed the effective performance of the prepared photocatalyst in the removal of drugs. Likewise, reuse and regeneration have an important role to play in reducing the economic cost and secondary pollution, as the Pd/ZnO nanocomposite has a good ability to regenerate compared to zinc oxide, with a high percentage (92.25% to 82.87%) of four cycles.
Graphical Abstract
Keywords
Main Subjects
Introduction
The influence of pollutants in wastewater on the aquatic environment is among the main topics that have been studied in recent years due to the release of effluents of pharmaceutical pollutants into wastewater. Its presence in water can interact with some other compounds and change its physical and chemical properties to more dangerous compounds. Many studies have been conducted using different metal oxides. However, titanium dioxide nanoparticles (TiO2 NPs) and Zinc Oxide nanoparticles (ZnO NPs) have been usually utilized either as semiconductors or nanoparticles in several applications especially in wastewater treatment [1-6]. There are many semiconductor photo catalysts such as ZnO, ZnS, CdS, CdSe, TiO2, etc. and have found widespread interest for the removal of many pharmaceuticals in water. Among the most important semiconductors whose photocatalytic properties have been studied, zinc oxide disks are the most important and widely used, because they are effective, environmentally friendly, inexpensive, stable, and harmless [7-12].
Zinc Oxide is one of the most important metal oxides and is considered one of the most exploited n-type semiconductors, which has been given priority due to its multifunctionality and is used in various applications. Metal oxide can also be manufactured by hydrolysis, pyrolysis, precipitation, electrophoresis, hydrothermal treatment, chemical methods, and gelation processes [13-16].
Sulfadiazine Hydrochloride (SFD), also known as 4-amino-N-2-pyrimidinylbenzenesulfonamide with the chemical formula C10H10N4O2S (refer to Figure 1), is an antibiotic belonging to the sulfonamide group. It is used to treat various infections such as trachoma, urinary tract infections, systemic bacterial infections, ocular toxoplasmosis. Sulfadiazine is included in pharmacopeias and may contain non-pharmacopeias impurities [17-19].
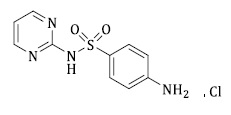
Figure 1. Chemical structure sulfadiazine hydrochloride (SFD) drug
Experimental
All chemicals used in the preparation of ZnO nanoparticles was obtained by a hydrothermal process, and the metal for (Pd) loaded onto ZnO nanoparticles was obtained by photochemical precipitation, as presented in Table 1. Deionized water was used in all experiments.
Synthesis of ZnO nanoparticles by hydrothermal method
Hydrothermal method was used to prepare zinc oxide nanoparticles by three stages. Stage one: zinc acetate dehydrate 30 g was added to 150 ml of distilled water and stirred for 60 min at 60 °C. Stage two: 30 g of oxalic acid 30 g of was dissolved in 150 ml of distilled water and stirred for 60 min. Stage three: The mixture of the solution on magnetic stirred for 10 min with heating at 65 °C and become a solution white color. After that, the solution was transferred in to autoclave and maintained in an oven at 160 °C for 24 h. The product was washed several times with a water to reach pH 7, dried at 70 °C for 24 h, and powder (white) was calcined at 400 °C for 1 h. Finally, powder (white) nanoparticles was used in all experimental.
Photochemical deposition of Pd/ ZnO nanocomposite
To deposit the Pd doped ZnO NPs, Nano-powder ZnO 0.5 g and PdCl2) 0.05 wt % was placed in a quartz cell in 100 ml of (methanol/ distilled water V/V 1%). The solution mixture was irradiated for 24 h with continuous stirring under ultraviolet light (major wavelength 365 nm, L.I. 2.3 mW/cm2). The powder was washed several times in distilled water and dried at 50 °C for 24 h, to obtain the Pd /ZnO nanocomposite.
Photocatalytic activity
Photo degradation experiments were conducted under optimal conditions to test different concentrations of the drug (10-50 mg/L), several weight of nanocomposite (0.1-0.4 g) using UV light at 365 nm. A weight of nanocomposite about 0.3 g was dispersed in 200 ml of the drug at a concentration of (20 mg /L). Before the beginning of the irradiation process, the experiment was conducted under the same conditions as the experiment, but without the use of light (dark), with stirring for 10 minutes. After that, the experiment was conducted at certain irradiation periods in the presence of light. About 3 ml of the solution was withdrawn from the suspension, and then centrifuged. The sample was measured using a UV-Vis spectrophotometer. The efficiency of the photolysis process was calculated with the Equation (1).
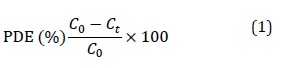
Table 1. The chemicals and materials utilized in this work
|
No. |
Chemicals |
Chemical formula |
Company |
Purity (%) |
|
1 |
Methanol |
CH3OH |
Sigma |
98.8 |
|
2 |
Sodium hydroxide |
NaOH |
BHD |
98 |
|
3 |
Zinc acetate dehydrate |
C4H6.O4Zn·2H2O |
Sigma |
98.5 |
|
4 |
Oxalic acid |
(COOH)2·2H2O |
BHD |
99.5 |
|
5 |
Palladium chloride |
PdCl2 |
Sigma |
99.1 |
|
6 |
Zinc oxide |
ZnO |
Sigma |
97.9 |
Where, PDE% is percentage of removal; C0 is the initial concentration; and Ct is the residual concentration after a selective time of degradation of drug under studying.
Results and Discussion
Field emission scanning electron microscopy (FE-SEM)
Images FE-SEM of zinc oxide nanoparticles and Pd/ZnO nanocomposite are demonstrated in Figure 2a. Image for ZnO NPs which was prepare by hydrothermal method that properties of agglomeration surface of ZnO NPs [20]. Therefore, after loading Pd onto ZnO the surface will be highly active site which led to create a suitable position for freely behavior of particles to produce nano rode shapes instead of spindle particles [21], as illustrated in Figure 2b.
Transmission electron microscopy (TEM)
Image TEM was found to limit the morphology, crystal structure and particle size. As appear the average size at 100, and 200 nm of ZnO NPs and Pd/ZnO nanocomposite, as shown in Figure 3a and b. The surface morphology and crystal structure of ZnO NPs and Pd/ZnO nanocomposite are depicted in Figure 3 at the surface of ZnO NPs in a white spherical structure [22]. In addition, after the process of loading Pd on ZnO NPs , a black spherical structure formed, evidence of loading Pd on the surface of zinc oxide nanoparticle The appearance of white spherical crystals in zinc oxide is evidence of the purity of the prepared compound. Furthermore, after the process of loading Pd on ZnO NPs, a black spherical structure formed, evidence of loading Pd on the surface of zinc oxide nanoparticle and the addition of the noble elements to zinc oxide is characterized by the property of crystallization in the form of spherical black crystals [23], as indicated in Figure 3d.

Figure 2. Image of FESEM of a) ZnO NPs and b) Pd/ZnO nanocomposite
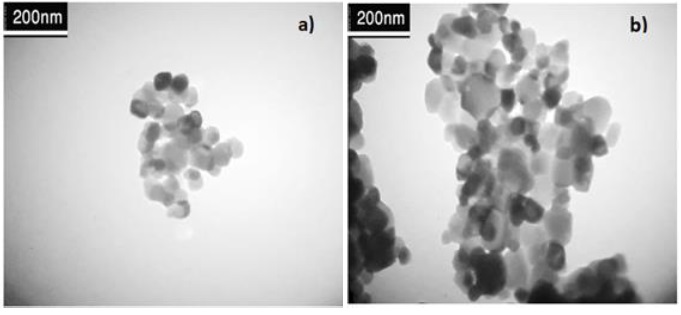
Figure 3. TEM Image of a) ZnO NPs and b) Pd/ZnO nanocomposite
Effect of mass Pd/ZnO nanocomposite
The influence of weight nanocomposite onto photo catalytic degradation of SFD drug, was studied utilizing 20 mg/L of SFD drug, flow rate of air 10ml/min, at 25 ᵒC, Figure 4 shows several weights of nanocomposite have been tested via the photocatalytic degradation method of DFS drug, as the weight of nanocomposite increases until reach to 0.3 g /200 mL photo degradation of DFS drug gradually rises, and then constant of photo catalytic efficiency. This may be attributed to the light absorption will be limited only to the first layers of drug and the other layers solution do not receive light photons [24, 25]. A nanocomposite weight of 0.3 g resulted in the highest photo degradation efficiency of 92.25%.
Effect of several concentrations
Effect of several initial concentration of DFS drug (10-50 mg/L) on photo degradation method of DFS drug was studied utilizing 0.3 g/200 mL, the light intensity of 1.3 mW/cm2 at 25 ᵒC. It has been observed that the rate of photo catalytic degradation decreases gradually with the increase of concentration of drug. This behavior could be explained, the concentration of DFS drug 20 mg/L was the optimum concentration to cover the largest area of nanocomposite. An important reason for this behavior is the high absorption of light by DFS drug present in the solution with a 20 mg/L concentration, the photocatalytic process occurs on the surface of a nanocomposite with 0.3 g/200 mL. Increasing the concentration of a drug prevents the penetration of light into the solution surface through successive layers of the drug on a nanocomposite surface [26-28]. A drug concentration 20 mg/L gives the optimum photolysis efficiency 92.25%, as displayed in the Figure 5.
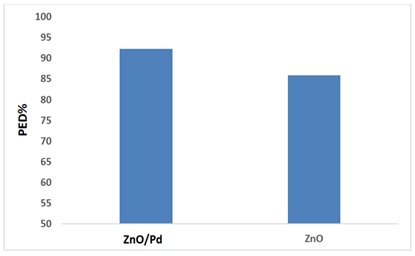
Comparison photo degradation between ZnO NPs and, Pd/ZnO nanocomposite
A comparison between surface, ZnO NPs and Pd/ZnO nanocomposite, according to the results, there is a clear difference in the results of photo catalysis between nanocomposite photo degradation efficiency PDE% 92.25% Pd/ZnO nanocomposite and photo degradation efficiency PDE% of ZnO NPs 85.8% to degradation of drug.
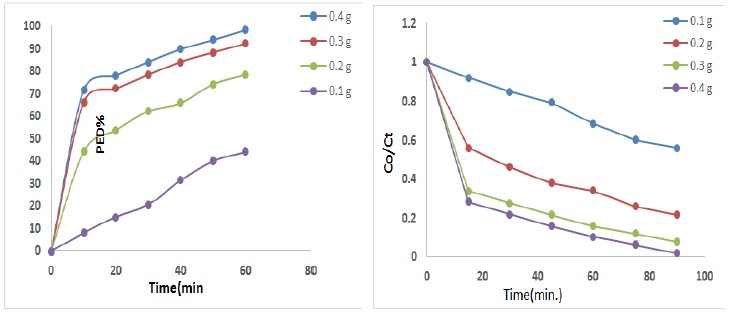
Figure 4. Effect of mass Pd/ZnO nanocomposite onto photo degradation efficiency of DFS drug
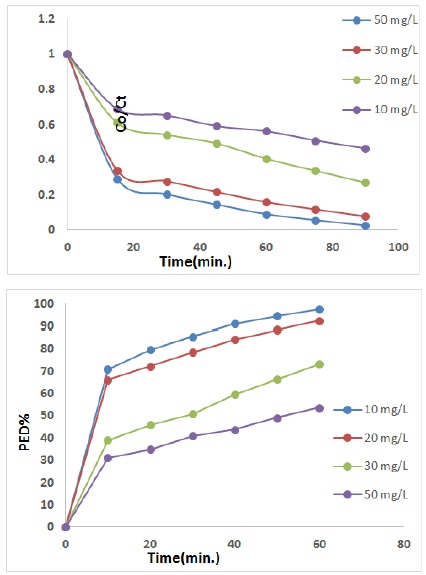
Figure 5. Photo catalytic degradation at several initial concentrations of DFS drug
It was clear from the results that with a photo catalyst (Pd/ZnO nanocomposite or ZnO NPs ) there was a very clear increase in the photocatalytic process, so that it had a clear effect compared to the absence of a catalyst [7, 23, 29], as shown in Figure 6.
Regeneration of ZnO NPs and Pd/ZnO nanocomposite
To reduce the economic cost using an environmentally friendly surface that is easily prepared from available, inexpensive materials, so it can be used more than once to remove pollutants because of the surface’s high efficiency and active sites that can be reactivated. The prepared surface ZnO NPs and Pd/ZnO nanocomposite has the photocatalytic efficiency of a drug within experimental conditions. The recyclability of ZnO NPs and Pd/ZnO nanocomposite was examined for four cycles, as shown in Figure 7 [24, 30]. The ZnO NPs and Pd/ZnO nanocomposite was washed several times in distilled water until the contaminant was removed from the surface. Based on the results, the photo catalysis gradually decreases after each cycle until all the surface active sites are saturated with the contaminant so that they cannot be removed and activated again. It was observed that the photocatalytic activity of the drug was (34.86%-82.34%) and (85.9%, 65.6%) for ZnO NPs and Pd/ZnO nanocomposite. This means that the surface can be used to remove pollutants with its advantage of reactivation and reduced economic cost [24, 28].
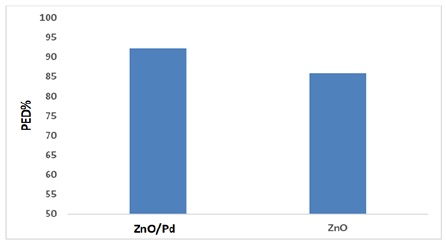
Figure 6. Comparison between surface, ZnO NPs and Pd/ZnO nanocomposite
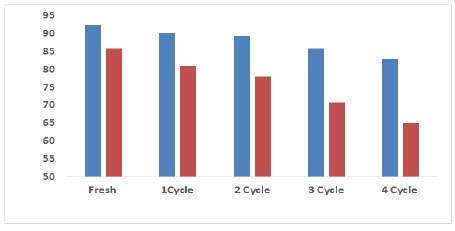
Figure 7. Regeneration of ZnO NPs and Pd/ZnO nanocomposite
Conclusion
This study is based on the preparation of two surfaces: First, the synthesis of ZnO nanoparticles by hydrothermal method and second, the photochemical deposition of Pd/ZnO nanocomposite. The highest photocatalytic degradation, which was achieved via utilizing Pd co-doping zinc oxide nanoparticles, reaches about 95.67% at 25 °C and ultimately can be used as a degradation DFs drug from an aqueous solution. The result is that when the weight of the nanocomposite increases, the photo degradation of the DFS drug gradually increases. An increase in the concentration of the drug decreases photo degradation because increasing the concentration of a drug prevents the penetration of light into the solution surface through successive layers of the drug on the nanocomposite surface. It was observed that reactivation was (34.86%,-82.34%) and (85.9%, 65.6%) for ZnO NPs and Pd/ZnO nanocomposite. This means that the surface can be used to remove pollutants, with the advantage of reactivation and reduced economic cost.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Orcid
Rathab Ali Ahmed
https://orcid.org/0009-0002-7479-3333
Ola Hamad Salah
https://orcid.org/0000-0002-3194-1749
Russul Reidh Abass
https://orcid.org/0000-0002-3697-3926
Manal Morad Karim
https://orcid.org/0000-0003-0061-0389
Shahad Abdulhadi Khuder
https://orcid.org/0009-0004-3760-4489
Salam Ahjelf
https://orcid.org/0009-0008-8881-6022
Imad Ibrahim Dawood
https://orcid.org/0000-0003-1636-7205
How to cite this manuscript: Rathab Ali Ahmed, Ola Hamad Salah, Russul Reidh Abass, Manal Morad Karim, Shahad Abdulhadi Khuder, Salam Ahjel, Imad Ibrahim Dawood. Improved Photocatalytic Degradation of Pharmaceutical Compounds from Aqueous Solutions Using Pd/Zno Nanocomposite as a Model of Environmental Applications. Asian Journal of Green Chemistry, 8(3) 2024, 308-318. DOI: 10.48309/AJGC.2024.449143.1487