Document Type : Original Research Article
Authors
- Rathab Ali Ahmed 1
- Ola Hamad Salah 2
- Haider Falih Shamikh Al-Saedi 3
- Manal Morad Karim 4
- Shahad Abdulhadi Khuder 5
- Anaheed Hussein Kareem 6
- Fathi Jihad Hammady 7
1 Department of Medical Engineering, AL-Nisour University College, Baghdad, Iraq
2 Department of Medical Engineering Al-Manara College for Medical Sciences, Maysan, Iraq
3 Faculty of Pharmacy, Department of Pharmaceutics, University of Al-Ameed, Iraq
4 College of Pharmacy, National University of Science and Technology, Dhi Qar, Iraq
5 Department of Medical Engineering, Al-Hadi University College, Baghdad,10011, Iraq
6 College of Health and Medical Technology, Al-Ayen University, Thi-Qar, 64001, Iraq
7 Department of Medical Engineering, Mazaya University College, Iraq
Abstract
In this study, the prepared, characterization, and photo-catalytic performance of zinc oxide/activated carbon (ZnO/AC) nanocomposites prepared via hydrothermal process to be applied for advanced oxidative process (AOPs). The ZnO/AC nanocomposites was characterized via field emission scanning electron microscopes (FE-SEM), transmission electron microscopy (TEM) and energy dispersive X-ray analysis (EDX) analyses. Different parameters were utilized to achieve best conditions including, weight of nanocomposite, and concentration of Riboflavin drug. Likewise, the photo-degradation appear high efficiency and activity when reused 5 cycles and confirm results that this photo-catalyst has promising prospects and a high ability to remove pollution from aqueous solution. Furthermore, AC can be a realistic and affordable re-placement for pricey noble metals. Photocatalytic activities of the catalytic adsorbents are used as model pollutant (Riboflavin drug) under UV irradiation. ZnO/AC nanocomposites showed excellent photo-catalytic activity (~99% degradation of drug in 60 min) compared with that of bare ZnO NPs and AC. In addition, a recycle or reused experiment demonstrated the best stability of the nanocomposite; the ratio photo-degradation of ZnO/AC reached last more 70% after five cycle successive runs and possessed strong photo-catalytic ability. The improve photo-catalytic activities may be related to the effects of the relatively high surface area. The best data between the studied photo-catalysts appear the drug removal efficiency of ∼92% in 1 h under UV light irradiation.
Graphical Abstract
Keywords
Main Subjects
Introduction
Every day, the environment is exposed to new pollutants from human activities such as farming, industry, healthcare, and household cleaning [1]. In the past twenty years, the presence of these contaminants has raised significant concern because of their harmful impacts. Common examples of emerging contaminants include pharmaceuticals, flame retardants, personal care products, and endocrine-disrupting [2].
Pharmaceuticals play a vital role in maintaining public health and improving quality of life [3]. A healthy society requires access to advanced medications and timely availability. Pharmaceutical use to treat various diseases has dramatically increased. Regrettably, the improper usage of medication has been common [4, 5]. Pharmaceuticals have proven effective in preventing and treating diseases in both humans and animals, but their residues are being released into the environment [6, 7]. Injecting pharmaceuticals directly into the soil as antibacterial agents for plant protection can also lead to a significant increase in the release of pharmaceuticals into water systems via surface. Most of the dose that the body does not absorb is released into the environment through human waste discharges. Common sources of pharmaceutical discharge into the environment are wastewater treatment plants, hospital effluents, septic tanks, surface waters, landfills, sweat, urine, and feces [8, 9]. Pharmaceuticals in the environment have been found to deteriorate both surface and subsurface water quality, ultimately harming the health of various organisms. Studies have shown that chronic, long-lasting, and sub-lethal effects of pharmaceuticals on aquatic populations and other non-target creatures have been documented [10].
The removal of pharmaceutical pollutants from wastewater are not effectively removed using traditional techniques, such as adsorption, coagulation, ultrafiltration, ion exchange synthetic, chemical oxidation, photocatalytic degradation, and electrochemical methods [11]. Due to the pollutants' synthetic origin and the presence of complex aromatic structures, to effectively remove the organic substrates, it is necessary to use safe products or methods that are less harmful to protect our environment heterogeneous photo catalysis, known as advanced oxidation processes. One of the methods for the degradation of harmful organic contaminants from air, soil and water [12-16]. These oxidation processes are cost-effective technologies that generate non-selective active species capable of oxidizing a wide range of non-biodegradable compounds. They have proven to be highly promising for effectively treating pollutants, whether in high or low [17, 18]. Riboflavin, also name known vitamin B2, Riboflavin is a dietary supplement in and also found in food, riboflavin is solid crystalline powder an orange-yellow and soluble in water. Chemical formula C17H20N4O6, molar mass 376.369 g·mol-1, and chemical stretcher as show in Scheme 1.
Heterogeneous photocatalytic is one of the advanced oxidation processes used for removal of Pharmaceutical and organic pollutant, in this type, the reactant and semiconductor will be found in different phase. The photo catalysis is a process in which semiconductor absorbs light energy to form radicals in the solution, these radicals are capable of oxidizing or reducing destroying the target contaminants. The oxidizing species, either bound hydroxyl radicals or free holes [19-22] , are generated as displayed in Figure 1.
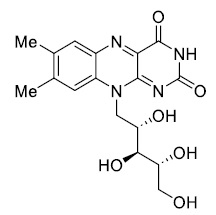
Scheme 1. Chemical stretcher of Riboflavin
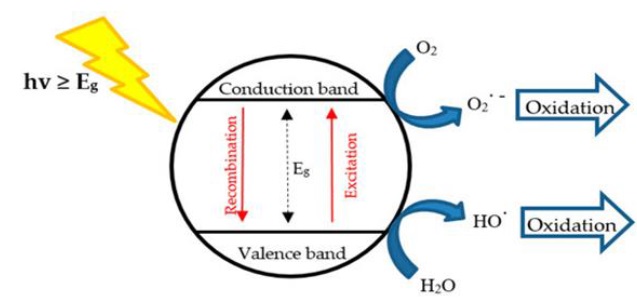
Figure 1. General important events that take place on an irradiation semiconductor particle [23]
Semiconductor types, such as TiO2, ZnO, CdS, and ZnS, can act as sensitizers for light-induced redox processes because of the electronic structure of the metal atoms in the chemical combination [24-26]. This is demonstrated by the filled valence band (VB) and an empty of a conduction band (CB). For a substance to be regarded as a semiconductor, the valence band and conduction band must both be separated by an energy gap, or band gab. When a semiconducting molecule absorbs photons with energy equal to or greater than its band-gab, electrons in the valance band can be excited and then jump up into the conduction band and thus charged carrier are generated [27].
Activated carbons (AC) as the utmost suitable, eco-friendly safe carbonaceous material for immobilization with semiconductor photo-catalysts. The high surface area of activated carbon enables powerful adsorption attraction for the elimination of pollutant during the photo catalytic method [28-31]. Here, prepared ZnO NPs decorated onto activated carbon (AC) via eco-friendly hydrothermal method [32, 33]. In this work, unique ZnO/AC nanocomposite was prepared utilized a facile, simple, eco-friendly, and cost-effective hydrothermal proses. Prepared samples were characterized utilizing FE-SEM, TEM, and UV-Vis, techniques, and evaluated via the photo-catalytic degradation of drug under UV irradiation.
Experimental
Preparation of fruit shells as activated carbon (AC)
Fruit shells were cleansed separated manually and wash distilled water to remove impurities, wiped dry in sun for three days. Activation method used hydrochloric acid (0.1N). After that, the mixture was put in furnace at temperature 300 °C for 2 h. The removal of acid by washing in distilled water until reached pH 7. The AC was then dried for 24 h at 110 °C in oven, the gathered AC was ground and filtered through a mesh 25 nm.
Preparation of ZnO/AC nanocomposite
Nanocomposite was prepared via utilizing a hydrothermal method. 5 g of Zinc acetate; 7 g of oxalic acid, and 0.5 g of AC was mixed, then complete to 150mL with distilled water then mixed for 30 min. to get a required solution. The resultant mixtures were kept at 160 oC for 24 h in an autoclave. The obtained powder dark brown, washed with distilled water then then dried at 60 oC for 24 h.
Photocatalytic activity
Photocatalytic degradation of the solution drug was prepared and stirred in dark for 10 min. (adsorption). ZnO/AC nanocomposite (0.3 g) was then added to the solution drug in order to catalyze the photo catalytic activity. The degradation method was continued for 1hr in order to achieve complete removal. Samples were removed at regular time intervals of 10 min in order to measure the decrease in drug concentration. The absorbance and drug concentration were checked utilizing a UV-Visible Spectrophotometry at (λmax = 545 nm) of drug. The drug chosen for degradation was drug at 50 mg/L .and photo catalytic degradation efficiency (%PDE) of drug was obtained utilizing Equation (1) as follows:
![]()
Results and Discussion
Characterization
FE-SEM image was utilized to study the morphology of ZnO/AC nanocomposite. The AC is agglomerated and the particle length 10 µm also the ZnO/AC nanocomposite i.e. is 10 µm. The morphology of surface of AC and ZnO/AC is shown in Figure 2a and b. AC nanoparticles were arranged in a flower-like pattern Figure 2a. These pores offered a strong opportunity for AC to get entangled within them. Figure 2b demonstrate SEM representations of ZnO/AC nanocomposites. ZnO nanoparticles were evenly distributed over activated carbon/brick grain fragments [34]. About the fact that ZnO immobilization on activated carbon/BG partially ZnO particle aggregation was significantly decreased in ZnO-AC with increased ZnO particle distribution over ZnO-AC/BG, according to SEM photos [35].
Figure 3 shows the TEM analysis of the surface morphology of the ZnO/AC nanocomposite. As depicted in Figure 3, the cloud became more prominent and a new design was formed after adorning the AC onto the surface of ZnO. This phenomenon is likely influenced by the quantity of carbon present on the surface of ZnO. TEM results provide insight into the structural composition of the ZnO/AC material as it is prepared.
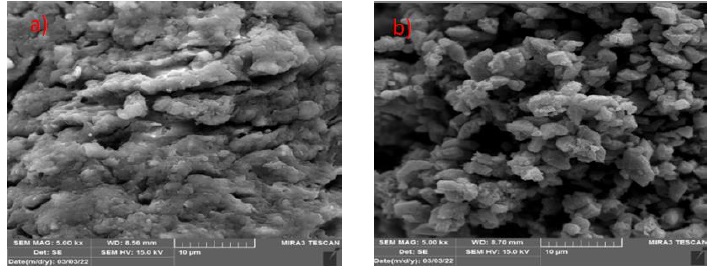
Figure 2. FE-SEM image a) AC surface, and b) ZnO/AC nanocomposite
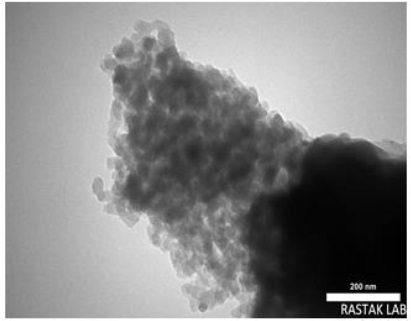
Figure 3. TEM image of ZnO/AC nanocomposite
The nanostructure resembling architecture consists of numerous individual single-crystal nano-plates, illustrated in Figure 2a and b. In addition, particles exhibit disordered wormhole-like pores, indicating a mesoporous structure [36]. Also, the TEM image of ZnO/AC showed a fine dispersion of black ZnO particles on the gray AC surface [37].
The EDX spectra of the ZnO/AC nanocomposite are presented in Figure 4. This analysis has verified the presence of the elements within the ZnO/AC nanocomposite. It can be seen from Figure 4 that Zn (76%), O (13.2%), and C (10.8%) elements have been detected in ZnO/AC nanocomposite.
Effect of weight ZnO/AC nanocomposite
The effect of several mass of ZnO/AC nanocomposite from 0.1-0.4 g on the photo catalytic degradation of Riboflavin drug was study at concentration drug 50 mg/L, light intensity (2.1 mW/cm2), flow rate of O2 (5 mL.min-1) and pH 6. The experimental result as shown in Figure 5. From data indicates that efficiency of photocatalytic degradation increases via increasing the quantity of ZnO/AC nanocomposite up to 0.3 g. Furthermore 0.3 g, the efficiency photo degradation was low increased or decreased slightly with increase weight of ZnO/AC nanocomposite. In the region less than 0.3 g, when the weight of ZnO/AC nanocomposite was increased, the efficiency of photo-degradation was increased because increase number of active sites [38, 39].
Effect concentration of riboflavin drug
The effect concentration of Riboflavin drug has been studied at mass of catalyst 0.3, light intensity (2.1 mW/cm2), solution pH, flow rate of O2 (5 mL.min-1) and concentrations of Riboflavin drug (20-90 mg/L). The experimental result as shown in Figure 6. Based on the result, when the concentration increase, the efficiency of photocatalytic degradation decreased, this happens via either reduced of the holes or (•OH) because the active sites will complete coverage via molecules of drug, or an raising in the concentration caused to rise adsorption of molecules drug on the surface ZnO/AC nanocomposite, which lead to reduce in the OH radical generation, because very little of active site obtainable as free on the catalyst surface [40,41].
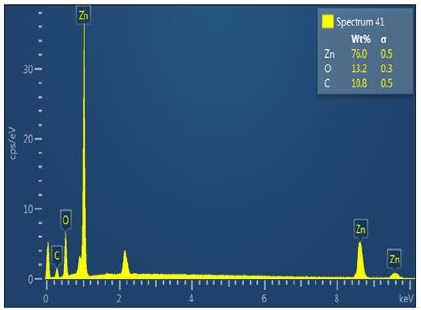
Figure 4. EDX analysis of ZnO/AC nanocomposite
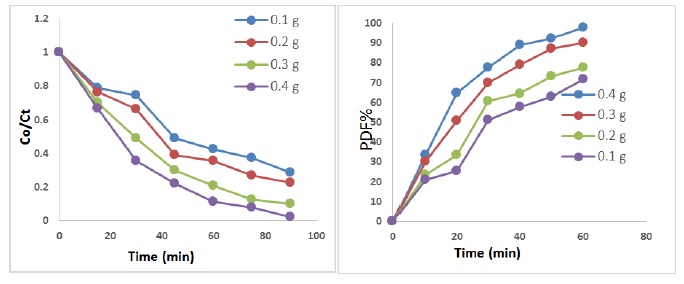
Figure 5. Photo-catalytic degradation onto drug at different weight of ZnO/AC nanocomposite
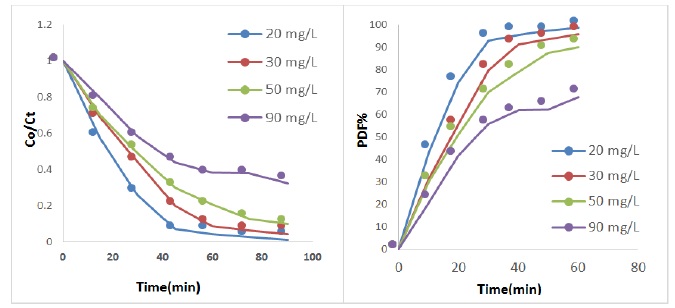
Figure 6. Photo-catalytic degradation at different concentration of Riboflavin drug
Regeneration /recycling of ZnO/AC nanocomposite
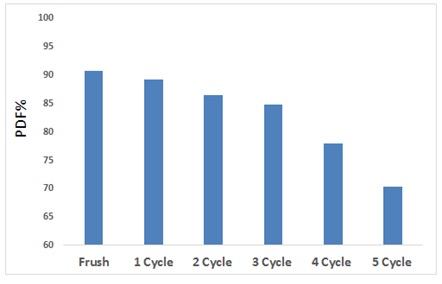
To reduce the economic cost via utilizing an ecofriendly surface that is easily syntheses from available, low-cost materials, so it can be utilize more than once to get rid of contaminants because of the surface’s high efficiency and active sites that can be reactivated. The reused ZnO/AC nanocomposite is one of the significant steps in assessing the practical applied of photo catalysts and in developing heterogeneous photo activity method for waste water treatment. According to Figure 7, an examination of the photocatalytic activity of the regeneration of ZnO/AC nanocomposite was carried out onto drug. The photo catalytic degradation efficiency was 90.9, 88.8, 86.5, 77.55, 82.43, and 70.15% for 5 cycle compared to standard solution (fresh) was 90.9%. The data of photo catalysts is effective, and thus the photo catalyst is basically stable and is therefore promising for environmental remediation [42, 43].
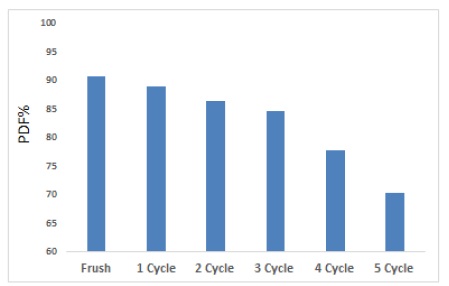
Figure 7. Regeneration /Recycling of ZnO/AC nanocomposite
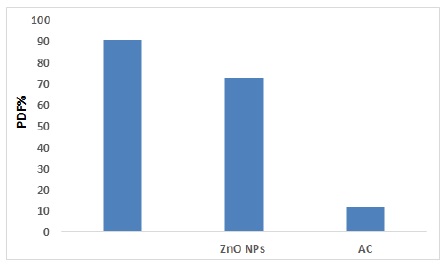
Figure 8. Comparison photo degradation among ZnO/AC nanocomposite, ZnO NPs and AC
Comparison photo degradation among ZnO/AC nanocomposite, ZnO NPs and AC
A comparison among nanocomposite surface, ZnO NPs and AC, according to the results, there is a clear difference in the results of photo catalysis between nanocomposite photo degradation efficiency PDE% 90.77% and ZnO/AC nanocomposite and photo degradation efficiency PDE% of ZnO NPs 70.8% to degradation of drug. But when use AC that appear very low removal of drug, the photo degradation efficiency PDE% 12.3%. It was clear from the results that with a photo catalyst (ZnO/AC nanocomposite or ZnO NPs) there was a very clear increase in the photocatalytic process, so that a clear effect compared to the absence of a catalyst, as show in Figure 8 [44] .
Conclusion
The application of agri-wastes as activated carbon (AC)was found a best candidate as compared to metals in obtaining a higher removal of pollutant. The preparation of ZnO /AC nanocomposite heterogeneous photo-catalysts by hydrothermal method. The heterogeneous photocatalyst ZnO/AC was found to be higher effective as compared to ZnO NPs. Their used to removal drug was tested by ZnO/AC nanocomposite was the best photocatalytic depredation under solar irradiation 90.77%, as compared to ZnO NPs 70.8%. The structural photo-catalytic degradation of ZnO/AC nanocomposite with optimum initial drug concentrations, mass of nanocomposite was studies. The nanocomposite sample with best optimum concentration appear higher photo-catalytic degradation and drug degradation (90% in 60 min) compared with samples ZnO NPs and AC, thus, nanocomposite can be utilized repeatedly with most no any change in photo-catalytic degradation after five cycles. Such ZnO/AC with enhancement properties shows great potential to be used as an effective photo catalyst adsorbent and used in remove pollutants from aqueous solutions.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Orcid
Rathab Ali Ahmed
https://orcid.org/0009-0002-7479-3333
Ola Hamad Salah
https://orcid.org/0000-0002-3194-1749
Haider Falih Shamikh Al-Saedi
https://orcid.org/0000-0003-0862-9543
Manal Morad Karim
https://orcid.org/0000-0003-0061-0389
Anaheed Hussein Kareem
https://orcid.org/0009-0008-8881-6122
How to cite this manuscript: R. Ali Ahmed, O. Hamad Salah, H. Falih Shamikh Al-Saedi, M. Morad Karim, S. Abdulhadi Khuder, A. Hussein Kareem, F. Jihad Hammady. A Green Surface Prepared with Highly Applicable of Advanced Oxidative Processes AOPs for Removal of Riboflavin Drug Pollutant Remediation. Asian Journal of Green Chemistry, 8(3) 2024, 296-307.
DOI: 10.48309/AJGC.2024.444917.1479