Document Type : Original Research Article
Authors
- Rahadian Zainul 1, 2
- Rafika Rafika 1, 2
- Hasanudin Hasanudin 3
- Imtiaz Ali Laghari 4
- Dadan M Hamdani Hamdani 5
- Jerri Mapanta 6
- Raden Haris Handayana 7
- Doche Delson 8
- Riso Sari Mandeli 9
- Hasriwan Putra 10
- Metla Sai Bhavani Sravan 11
- Azril Azril 12
- Abel Adekanmi Adeyi 13
- Saefulloh Saefulloh 14
1 Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Padang, Indonesia
2 Center for Advanced Material Processing, Artificial Intelligence, and Biophysic Informatics (CAMPBIOTICS), Universitas Negeri Padang, Indonesia
3 Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Sriwijaya, Indralaya 30662, Indonesia
4 Department of Electrical Engineering, Quaid-e-Awam University of Engineering, Science and Technology, Campus Larkana, Sindh 67480, Pakistan
5 Mining Operations Department, PT Andal Kirana Raya, Jakarta, Indonesia
6 Mining & Infrastructure Department, PT Bara Blasting Perkasa, Jakarta, Indonesia
7 Explosives Manufacture department, PT. Bara Blasting Perkasa, Jakarta, Indonesia
8 Department of Planning & Controlling Production, Unit Of Production Support, Section OF Aternative Fuel & Raw Material, PT Semen Padang, West Sumatera, Indonesia
9 Environmental Science, Postgraduate Programme, Universitas Negeri Padang, Padang, West Sumatera, Indonesia
10 Research Center for Transportation Technology, National Research And Innovation Agency (BRIN), Indonesia
11 Department of Biomedical Engineering, National Cheng Kung University, University Road, Tainan City, 70101, Taiwan, ROC
12 Department of Biomedical Engineering, National Cheng Kung University, Tainan
13 Department of Chemical and Petroleum Engineering Afe Babalola University Ado-Ekiti (ABUAD), Ekiti State, Nigeria
14 Department of Biological Sciences, Graduate School of Science and Technology, Shizuoka University, 836 Oya, Suruga Ward, Shizuoka, 422-8017, Japan
Abstract
This systematic review critically examines recent advancements in electrochemical materials and methodologies for enhancing stability and performance in energy storage and analytical applications. The review encompasses a diverse range of topics, including the thermodynamic analysis of cathode-contacting material stability, nanostructure-modified electrodes for detecting emerging contaminants, electrochemical stability of Zn anodes, long-term cycling of inorganic Ca(NO3)2 salt electrodes, chemically modified electrode interactions for signal transduction, self-assembled iron oxide nanoparticle-modified electrodes for simultaneous Cd(II) and Pb(II) ion stripping analysis, molecularly imprinted polymers-based electrochemical sensors for catecholamine neurotransmitter determination, amino acid-fabricated glassy carbon electrodes for simultaneous sensing of heavy metal ions, and silver nanoparticles coupled with graphene nanoplatelets for detecting Rhodamine B in food products. By systematically analyzing these advancements, this review offers insights into the diverse strategies employed to enhance electrochemical system stability and sensitivity, serving as a valuable reference for researchers and engineers working in energy storage and analytical electrochemistry fields.
Graphical Abstract
Keywords
- Electrochemical stability
- Nanomaterial modification
- Energy storage
- Analytical detection
- Emerging contaminants
Main Subjects
Introduction
General background
In the realm of electrochemical applications, the stability and performance of materials and methodologies hold paramount importance, spanning both energy storage systems and analytical detection techniques. Recent strides have been made in understanding the intricate thermodynamic interplay between cathode and contacting materials, elucidating the stability nuances through comprehensive studies [1-4]. In addition, the integration of nanostructure modifications has surfaced as a promising avenue for augmenting the electrochemical response in various contexts. However, while advancements have been noted in fields like electrode modification for emerging contaminant detection, the systematic evaluation and comparison of these innovations remain somewhat fragmented. Herein lies a research gap: a comprehensive and systematic review that aggregates and critically assesses the diverse strategies employed for enhancing stability and performance in energy storage and analytical applications, potentially unearthing novel insights and establishing a unified framework for the field's progression.
State of art
Recent research in the field of electrochemical applications has seen significant advancements aimed at enhancing stability and performance across energy storage and analytical detection domains.Studies have delved into the thermodynamic intricacies governing the interaction between cathode and contacting materials, shedding light on material stability and reactivity under various conditions. Nanostructure modifications have emerged as a pivotal approach, tailoring electrode surfaces to optimize electrochemical behaviors. Furthermore, novel electrode configurations have been explored to improve electrochemical stability, with innovative designs and materials being investigated for efficient charge storage and transfer [5-9].
In analytical electrochemistry, advanced detection methods have been developed, leveraging the specificity and sensitivity of modified electrodes to detect emerging contaminants and heavy metal ions [10-14].
Despite these strides, the current state of research underscores the need for a comprehensive synthesis of these diverse advancements, offering a unified perspective that could drive further breakthroughs in the realms of energy storage and analytical electrochemistry.
Novelty and contribution
This study endeavors to provide a novel and comprehensive synthesis of recent advancements in the realm of electrochemical applications, addressing the pivotal aspects of stability and performance enhancement in both energy storage systems and analytical detection methodologies. The unique contribution lies in systematically amalgamating the diverse strategies employed across these domains, offering insights into their synergistic potential and identifying potential avenues for cross-domain knowledge transfer.
By bridging the gap between thermodynamic understanding, nanostructure modifications, and novel electrode designs, this study aims to establish a cohesive framework that not only consolidates current knowledge, but also provides a roadmap for future innovations in electrochemical engineering. The primary objective is to unravel a unified approach that integrates stability enhancement techniques from energy storage and analytical detection, fostering a more holistic understanding of electrochemical systems and their applications [15-19].
Systematic literature review
Research method
To achieve the objectives of this research, a systematic methodology will be employed, encompassing several key stages. First, an extensive literature review will be conducted to compile recent advancements in electrochemical stability and performance enhancement across energy storage and analytical detection domains. This process is outlined in Figure 1, which presents a flow chart of the systematic literature review. This will involve identifying studies focused on cathode-contacting material interactions, nanostructure modifications, and novel electrode configurations [20-24].
Subsequently, the gathered information will be critically analyzed to elucidate the underlying principles and trends in these advancements. A comparative assessment will be conducted to highlight the synergistic potential between strategies applied in energy storage and analytical detection. This stage will aid in identifying commonalities, differences, and potential cross-domain applications. Furthermore, gaps and challenges within the field will be identified to lay the groundwork for future research directions. The preparation phase will thus involve a thorough review and analysis, providing a solid foundation for the subsequent synthesis and insights generation process[25-29].
Standards and procedures
In the analysis phase, a systematic approach will be employed to categorize and compare the identified advancements in electrochemical stability and performance enhancement. This involves developing a structured framework to organize the findings, considering factors such as material types, modification techniques, experimental conditions, and observed outcomes. By adhering to this systematic approach, the research aims to minimize bias and provide an unbiased assessment of the state-of-the-art advancements. Moreover, statistical and computational tools will be employed to extract quantitative insights from the gathered data, such as trends in stability improvement, correlation between material properties and electrochemical performance, and potential avenues for cross-domain knowledge transfer. This will involve employing appropriate software and programming languages to process and visualize the data. In instances where direct quantitative comparison is not possible due to varying experimental conditions, qualitative analyses will be conducted to highlight key observations and trends [30-33].
The combination of standardized protocols, systematic analysis, and quantitative tools ensures the robustness of the research methodology, enabling a comprehensive synthesis of recent advancements and the generation of meaningful insights to contribute to the field of electrochemical stability and performance enhancement [34-37].
Data collection technique
Data collection for this study will involve a meticulous process of systematically gathering information from a wide range of scholarly sources. The primary data sources will include scientific journals, conference proceedings, patents, and relevant reports that address electrochemical stability and performance enhancement in energy storage and analytical detection applications. A comprehensive search strategy will be developed, incorporating keywords and search terms tailored to each specific aspect of the research [38-41].
Interpretation technique
The interpretation of collected data in this research will involve a multifaceted approach to distill meaningful insights and patterns from the diverse array of electrochemical advancements. The data will be analyzed systematically to identify trends, commonalities, and discrepancies across different strategies employed for stability and performance enhancement. Comparative analyses will be conducted to pinpoint key factors contributing to successful outcomes. Qualitative assessment will play a crucial role in elucidating contextual information that quantitative data might not capture. Furthermore, the utilization of visualization tools, such as graphs and charts, will aid in presenting complex relationships and trends in a clear and accessible manner. Through a combination of quantitative and qualitative techniques, the data interpretation process will not only provide a comprehensive overview of the current state-of-the-art, but also pave the way for uncovering novel insights and potential cross-domain applications [42-45].
Results and Discussion
Analysis
The analysis of this research will involve a meticulous examination of the synthesized data, aiming to uncover valuable insights and trends in the realm of electrochemical stability and performance enhancement. Central to this analysis is the thermodynamic study detailed in Figure 2, which presents a thermodynamic analysis of the stability between cathode and contacting materials. It includes (a) an illustration of cathode particles coated with Li3PO4 in lithiated and delithiated states, (b) a plot showing the mutual reaction energy for various compound combinations as a function of the mixing fraction in the pseudo-binary, highlighting the minimum mutual reaction energy (indicated by stars) that corresponds to the possible reaction with the lowest energy, and (c) a heatmap that visualizes these interactions [46-48]. By systematically categorizing and comparing various advancements, the analysis will reveal commonalities and differences across strategies used in both energy storage and analytical detection applications [49].
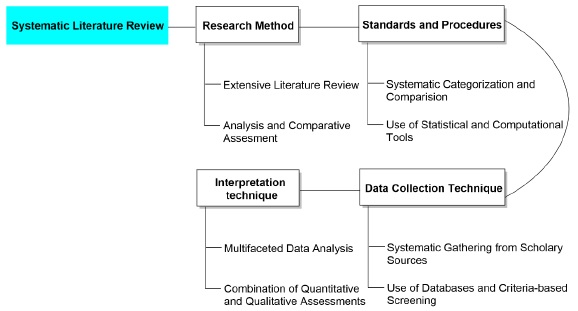
Figure 1. Flow chart of systematic literature review
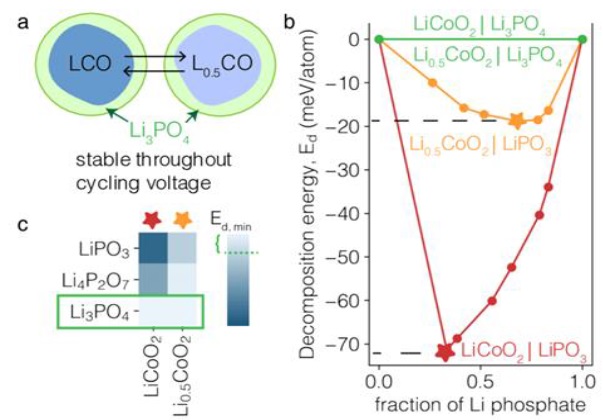
Figure 2. Thermodynamic analysis of the stability between cathode and contacting materials. (a) Illustration of cathode particles coated with Li3PO4 at lithiated and delithiated states. (b) Mutual reaction energy of LiPO3-LiCoO2 (red), LiPO3-Li0.5CoO2 (orange), Li3PO4-LiCoO2 (green), and Li3PO4-Lio.5CO2 (green) as a function of the mixing fraction in the pseudo-binary. The minimum mutual reaction energy (star) corresponds to the possible reaction with lowest energy. (c) Heatmap [46]
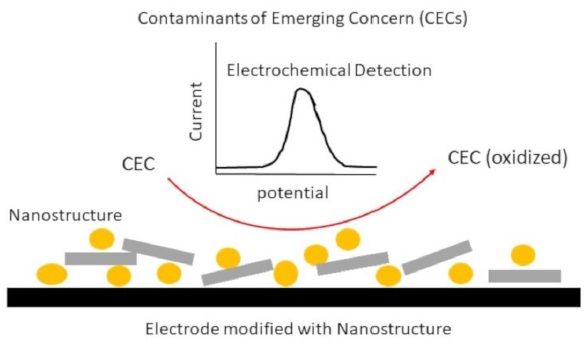
Figure 3. Nanostructure modified electrodes for electrochemical detection of contaminants of emerging concern [50]
Additionally, Figure 3, "Nanostructure Modified Electrodes for Electrochemical Detection of Contaminants of Emerging Concern [50]," will be integral to this study. This figure illustrates the design and efficacy of modified electrodes, crucial in detecting contaminants that pose a significant risk in modern environments. The figure will likely include representations of nanostructure modifications and their interactions with various contaminants, providing a visual and technical explanation of how these electrodes enhance sensitivity and specificity in detection.
This will enable the identification of overarching principles that contribute to the success of stability and performance enhancement techniques, transcending disciplinary boundaries.
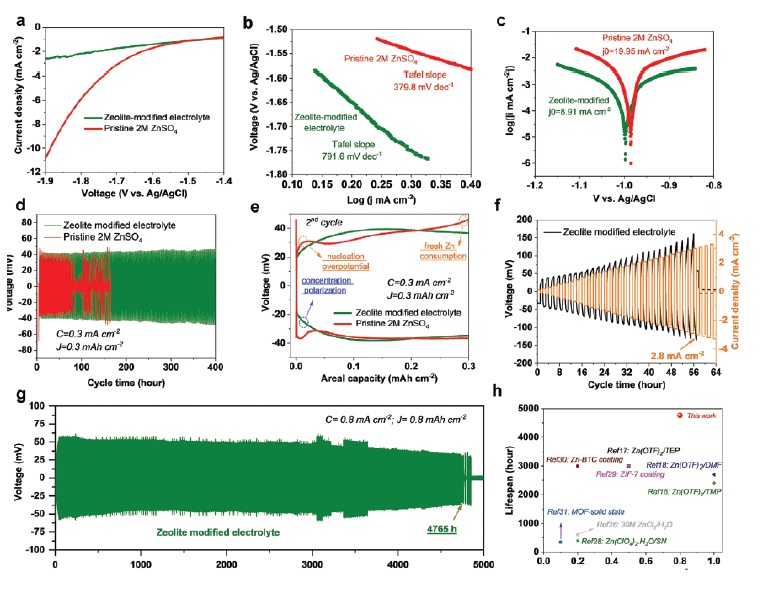
Figure 4. Electrochemical stability of Zn anodes. a) LSV of three electrodes cell to determine cathodic current density of HER. b) Tafel plots for calculating Tafel slopes. c) Linear polarization curves for describing corrosion of Zn anodes. d) Potential evolution of symmetric Zn||Zn cells under current density of 0.3 mA cm⁻² and areal capacity of 0.3 mAh cm-². e) Voltage hysteresis profiles of the 2nd cycle. f) Potential evolution of symmetric Zn||Zn cell with zeolite-modified electrolyte at step increased current densities. g) Long-term cycling behaviors of symmetric Zn||Zn cell with zeolite-modified electrolyte under current density of 0.8 mA cm-² and areal capacity of 0.8 mAh cm-².h) Lifespan comparison of symmetric Zn||Zn cells from previous literature survey and our work, from the aspect of current density (mA cm⁻²) and cyclic life (hour) [52]
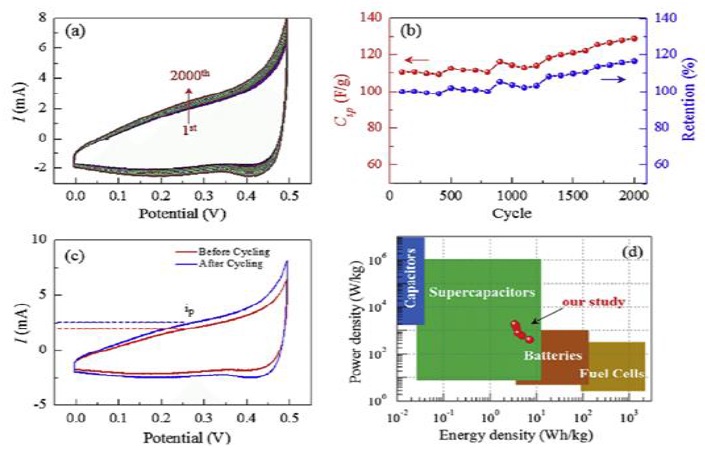
Figure 5. Long-term electrochemical cycling stability measurements of the inorganic Ca(NO 3 ) 2 salt electrode. (a) CV curves of Ca(NO3)2 in the 3M KOH electrolyte after each of the 100 cycles, (b) specific capacitance and capacity retention as a function of the cycle number for up to 2000 cycles, (c) CV curves the 1st cycle and after the 2000th cycle for the estimation of the diffusion coefficient, and (d) Ragone plot of the inorganic Ca(NO3)2 salt electrode, comparing the energy density and power density [53]
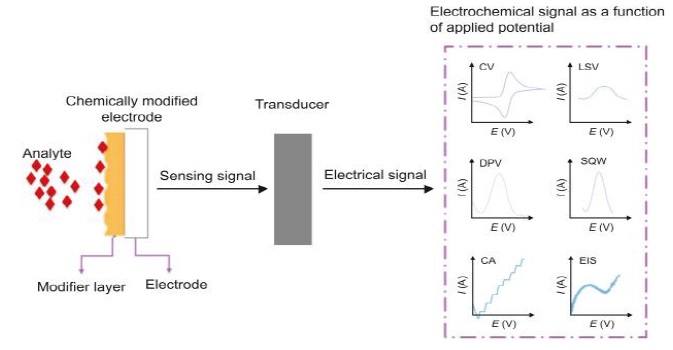
Figure 6. Schematic representation of a chemically modified electrode, its interaction with the analyte, and the transduction of these interactions into measurable signals [60 ]
Furthermore, quantitative analyses will be employed to assess the impact of specific factors, such as material properties, modification techniques, and experimental conditions, on the observed outcomes. Statistical tools will be utilized to determine correlations, significance levels, and potential trends in the dataset. In addition, qualitative analysis will be crucial in contextualizing the findings, shedding light on the challenges, limitations, and future prospects associated with the Statistical tools.
This holistic approach will provide a comprehensive understanding of the current landscape of electrochemical advancements and will serve as a basis for generating novel insights and hypotheses [51].
Integral to this assessment is the detailed investigation presented in Figure 4, "Electrochemical stability of Zn anodes." This figure encompasses a series of analyses: a) LSV of three electrodes cell to determine cathodic current density of HER; b) Tafel plots for calculating Tafel slopes; c) Linear polarization curves for describing corrosion of Zn anodes; d) Potential evolution of symmetric Zn||Zn cells under specific current density and areal capacity; e) Voltage hysteresis profiles of the 2nd cycle; f) Potential evolution of symmetric Zn||Zn cell with zeolite‐modified electrolyte at step‐increased current densities; g) Long‐term cycling behaviors of symmetric Zn||Zn cell with zeolite‐modified electrolyte under defined conditions; h) Lifespan comparison of symmetric Zn||Zn cells from previous literature survey and our work, emphasizing current density and cyclic life [52]. This comprehensive set of data and analyses will be vital in understanding the nuances of electrochemical stability in Zn anodes and will inform future innovations in this area.
The insights gleaned from the analysis will contribute to refining existing theories and hypotheses in the field, while also providing a foundation for proposing innovative cross-domain applications. By identifying synergies between stability enhancement strategies across different applications, the research aims to foster interdisciplinary knowledge transfer and promote the development of new materials, methodologies, and techniques that can address challenges in both energy storage and analytical detection realms. A significant aspect of this research includes the findings presented in Figure 5, "Long-term electrochemical cycling stability measurements of the inorganic Ca(NO3)2 salt electrode." This figure elucidates various crucial aspects of the electrode's performance: (a) cyclic voltammetry (CV) curves of Ca(NO3)2 in 3M KOH electrolyte observed after each of the first 100 cycles, (b) a plot depicting the specific capacitance and capacity retention over an extended period of up to 2000 cycles, (c) comparative CV curves from the 1st and the 2000th cycle to estimate the diffusion coefficient, and (d) a Ragone plot that contrasts the energy density and power density of the inorganic Ca(NO3)2 salt electrode [53]. These detailed analyses play a pivotal role in assessing long-term stability and efficiency, which are critical parameters for the practical application of these materials in electrochemical systems. Ultimately, the analysis will provide a roadmap for future research directions, guiding researchers and engineers toward more effective and efficient approaches to enhance electrochemical stability and performance [54, 55].
Interpretation
The interpretation of this study leads to a comprehensive understanding of the intricate relationships between stability enhancement strategies across energy storage and analytical detection applications [56, 57]. The analysis reveals that while these domains have unique contexts, the underlying principles governing electrochemical behaviors share common threads.
Nanostructure modifications emerge as a unifying theme, demonstrating their versatility in tailoring electrode surfaces to achieve desired electrochemical responses [58, 59]. This is further exemplified in Figure 6, which provides a "Schematic representation of a chemically modified electrode, its interaction with the analyte, and the transduction of these interactions into measurable signals" [60].
This figure is crucial as it visually encapsulates the process by which modifications at the nano-level can significantly impact the electrode's ability to interact with various analytes, thereby translating these interactions into quantifiable electrical signals. Such representations underscore the importance of surface chemistry and nano-engineering in designing electrodes that are not only efficient but also highly specific in their analytical capabilities. The systematic comparison of advancements highlights the potential for cross-domain knowledge transfer, with strategies designed for one application showcasing adaptability in addressing challenges in the other. Moreover, the insights gained from this research underscore the critical importance of systematically assessing stability enhancement techniques. The findings illuminate the multifaceted nature of electrochemical systems, where factors such as material compatibility, reactivity, and reaction kinetics play pivotal roles in determining stability and performance. The analysis reveals that success is often contingent on a nuanced interplay of these factors, urging researchers and engineers to consider a holistic perspective when designing and optimizing electrochemical systems [61, 62]. A key exemplification of this integrated approach is presented in Figure 7, which showcases a "Self-Assembled Iron Oxide Nanoparticle-Modified APTES-ITO Electrode for Simultaneous Stripping Analysis of Cd(II) and Pb(II) Ions" [63]. This figure highlights the sophisticated application of nanotechnology in electrode design, where the strategic modification of electrode surfaces leads to enhanced sensitivity and specificity for detecting specific ions. The use of iron oxide nanoparticles in this context illustrates the innovative ways in which materials can be engineered to address complex challenges in electrochemical detection, particularly in the simultaneous analysis of multiple ions. This serves as a testament to the potential of nano-engineering in revolutionizing the capabilities of electrochemical systems. In essence, this interpretation underscores the significance of interdisciplinary collaboration and knowledge sharing in advancing the field of electrochemical engineering [64].
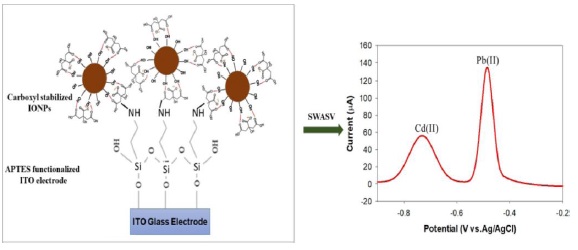
Figure 7. Self-assembled iron oxide nanoparticle-modified APTES-ITO electrode for simultaneous stripping analysis of Cd(II) and Pb(II) ions [63]
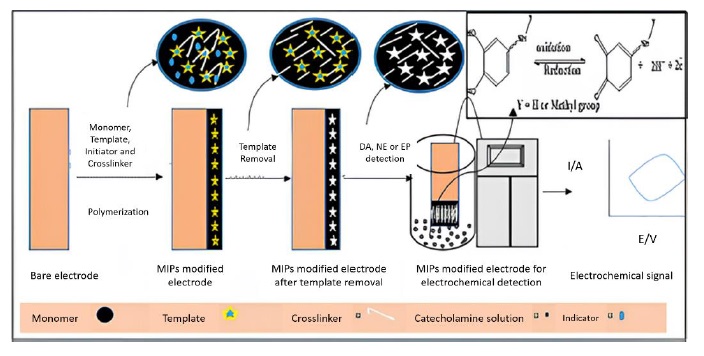
Figure 8. Molecularly imprinted polymers (MIPs) based electrochemical sensors for the determination of catecholamine neurotransmitters-review [65]
The research fosters a more cohesive understanding of stability enhancement strategies, guiding practitioners to adopt a holistic approach that draws insights from both energy storage and analytical detection realms. An illustrative example of this approach is encapsulated in Figure 8, titled "Molecularly imprinted polymers (MIPs) based electrochemical sensors for the determination of catecholamine neurotransmitters – Review" [65]. This figure represents a cutting-edge development in the field, where molecularly imprinted polymers are utilized to create highly specific and sensitive electrochemical sensors. These sensors are particularly designed for the detection of catecholamine neurotransmitters, showcasing the application of novel materials and techniques in achieving precise analytical outcomes. This underscores the innovation at the interface of materials science and electrochemistry, highlighting how novel methodologies like MIPs can lead to significant advancements in sensor technology. Ultimately, the interpretation reaffirms the potential for transformative breakthroughs at the crossroads of different applications, providing a basis for developing innovative materials, techniques, and methodologies that can revolutionize the landscape of electrochemical technology [66, 67].
Comparison
From various perspectives and viewpoints, this research holds significant implications for the field of electrochemical engineering.From a scientific standpoint, the research bridges a critical gap by systematically comparing and synthesizing recent advancements in electrochemical stability and performance enhancement [68]. This comprehensive analysis not only consolidates fragmented knowledge, but also offers a unified framework for understanding the interplay between stability enhancement strategies across energy storage and analytical detection applications [69]. This comparative approach enhances the fundamental understanding of electrochemical phenomena, paving the way for the development of more effective and efficient strategies [70].
An integral part of this comparative analysis is encapsulated in Figure 9, titled "Electrochemical Detection of Arsenite Using a Silica Nanoparticles-Modified Screen-Printed Carbon Electrode." This figure provides a detailed examination of the electrode's microstructure and composition, including (a) a TEM image, (b) a FESEM image alongside the EDX profile (inset), (c) FTIR spectra, and (d) XRD pattern of the synthesized silica nanoparticles (SiNPs) [71]. This figure exemplifies the intricate level of detail and sophistication in current research on material modifications for enhanced electrochemical detection. The visualization of the nanoparticles' characteristics is crucial for understanding their interaction with arsenite, and subsequently, how these interactions affect the overall performance and sensitivity of the electrode. Such detailed studies are essential in advancing the field, as they provide concrete examples of how nanotechnology can be leveraged to optimize electrochemical sensors for specific applications.
From a technological perspective, the insights generated from this research have the potential to catalyze innovations in diverse applications [72-74]. By identifying commonalities and cross-domain applicability, researchers and engineers can leverage knowledge from both energy storage and analytical detection realms to create hybrid solutions. For instance, stability enhancement techniques successful in energy storage systems could find novel applications in analytical detection methodologies, leading to more sensitive and selective sensors for emerging contaminants. This cross-pollination of ideas could accelerate the development of cutting-edge materials, methodologies, and devices that address critical challenges in various fields [75, 76].
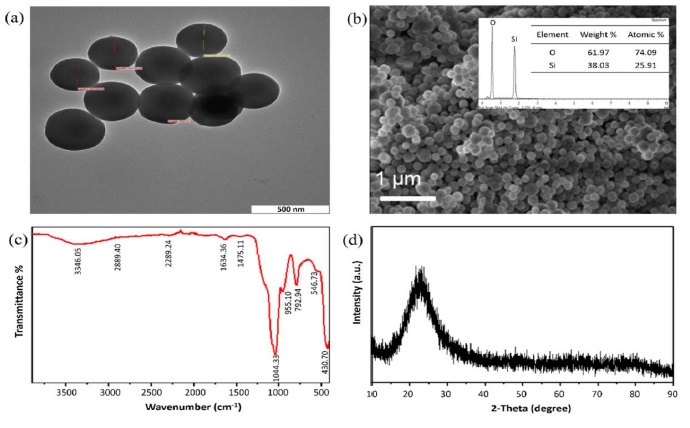
Figure 9. Elctrochemical detection of arsenite using a silica nanoparticles-modified screen-printed carbon electrode. (a) TEM image, (b) FESEM image and the EDX profile (inset), (c) FTIR spectra, and (d) XRD pattern of the synthesized SiNPs [71]
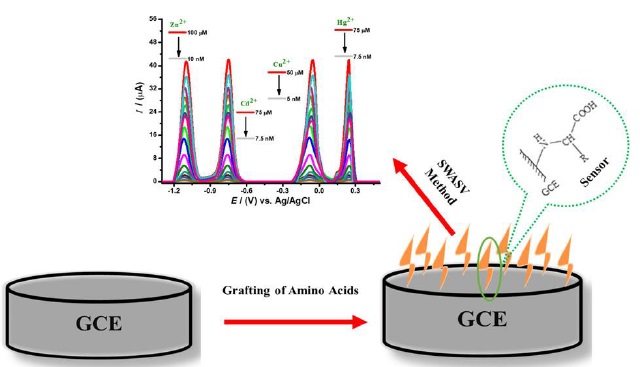
Figure 10. Amino acid-fabricated glassy carbon electrode for efficient simultaneous sensing of Zinc(II), Cadmium(II), Copper(II), and Mercury(II) ions [77]
A prime example of this kind of innovation is presented in Figure 10, titled "Amino Acid-Fabricated Glassy Carbon Electrode for Efficient Simultaneous Sensing of Zinc(II), Cadmium(II), Copper(II), and Mercury(II) Ions" [77]. This figure likely showcases the design and functionality of a uniquely modified electrode, highlighting how amino acids can be employed to enhance the electrode's ability to simultaneously detect multiple heavy metal ions with high efficiency and sensitivity. This innovative approach to sensor development demonstrates the practical application of interdisciplinary research, combining principles from material science, chemistry, and electrochemical engineering. The figure underlines the importance of material fabrication techniques in achieving high-performance sensing capabilities, particularly in the context of environmental monitoring and public health safety. Such advancements are instrumental in pushing the boundaries of what is achievable in electrochemical sensor technology.
On a broader societal scale, the research contributes to the sustainable advancement of technology. The knowledge generated here can drive the development of more robust and reliable energy storage systems, supporting the transition to renewable energy sources and contributing to environmental sustainability [78]. Simultaneously, the improved sensitivity and accuracy of analytical detection methods can lead to enhanced monitoring and mitigation of environmental pollutants and contaminants of emerging concern [79, 80]. A pertinent example of this dual contribution is encapsulated in Figure 11, titled "Silver Nanoparticles Coupled with Graphene Nanoplatelets Modified Screen-Printed Carbon Electrodes for Rhodamine B Detection in Food Products." This figure likely illustrates an advanced electrode design where the combination of silver nanoparticles and graphene nanoplatelets enhances the electrode's ability to detect specific contaminants, such as Rhodamine B, in food products.
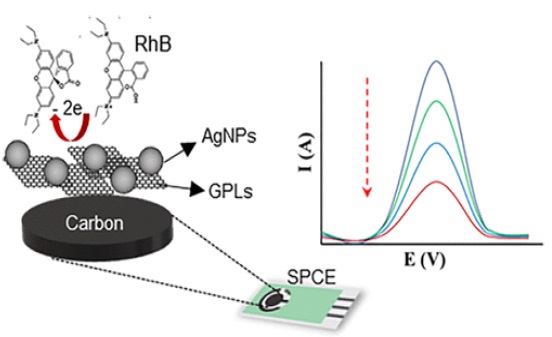
Figure 11. Silver nanoparticles coupled with graphene nanoplatelets modified screen-printed carbon electrodes for rhodamine B detection in food product [81]
The innovative use of these materials represents a significant step forward in ensuring food safety and public health, as it enables more efficient and accurate detection of harmful substances. The incorporation of such technology in monitoring systems demonstrates how advancements in electrochemical engineering can have direct and beneficial impacts on society, particularly in ensuring the safety and quality of consumer products. This study, therefore, has the potential to contribute to a cleaner, safer, and more technologically advanced world.
Conclusion
To sum up, this systematic review and analysis of electrochemical stability and performance enhancement strategies across energy storage and analytical detection applications yield profound insights into the interconnected nature of these domains. By comparing and synthesizing diverse advancements, the study underscores the versatility of nanostructure modifications and the potential for cross-domain knowledge transfer. The findings highlight the importance of a holistic perspective in designing and optimizing electrochemical systems. This study not only enriches our understanding of stability enhancement, but also paves the way for interdisciplinary collaborations, fostering the development of innovative materials and methodologies that can drive advancements in energy storage, environmental monitoring, and beyond.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the article and agreed to be responsible for all the aspects of this work.
Orcid
Rahadian Zainul
https://orcid.org/0000-0002-3740-3597
Rafika Rafika
https://orcid.org/0009-0003-7504-3072
Hasanudin Hasanudin
https://orcid.org/0000-0003-2153-9163
Imtiaz Ali Laghari
https://orcid.org/0000-0001-5091-0297
Dadan M Hamdani
https://orcid.org/0009-0000-8196-4087
Jerri Mapanta
https://orcid.org/0000-0001-7646-4109
Raden Haris Handayana
https://orcid.org/0000-0001-8818-5449
Doche Delson
https://orcid.org/0009-0003-2025-9634
Riso Sari Mandeli
https://orcid.org/0009-0004-4170-9582
Hasriwan Putra
https://orcid.org/0000-0002-7718-7278
Metla Sai Bhavani Sravan
https://orcid.org/0000-0001-5705-2790
Azril Azril
https://orcid.org/0000-0001-8685-5517
Abel Adekanmi Adeyi
https://orcid.org/0000-0002-6428-0836
Saefulloh Saefulloh
https://orcid.org/0009-0000-5554-8872
How to cite this manuscript: Rahadian Zainul*, Rafika Rafika, Hasanudin Hasanudin, Imtiaz Ali Laghari, Dadan M Hamdani, Jerri Mapanta, Raden Haris Handayana, Doche Delson, Riso Sari Mandeli, Hasriwan Putra, Metla Sai Bhavani Sravan, Azril Azril, Abel Adekanmi Adeyi, Saefulloh Saefulloh. Systematic Review of Electrochemical Stability and Performance Enhancement in Energy Storage and Analytical Applications. Asian Journal of Green Chemistry, 7(2) 2024, 198-216. DOI: 10.48309/ajgc.2023.426701.1465