Document Type : Original Research Article
Authors
- Khemchand Rajendra Surana 1
- Sagar V. Bhavar 1
- Vijayraj N. Sonawane 1
- Dhananjay M Patil 2
- Deepak D Sonawane 2
- Sunil K Mahajan 3
1 Department of Pharmaceutical Chemistry, SSS’s Divine College of Pharmacy, Nampur Road, Satana, Nashik, Maharashtra, India – 423301
2 Department of Pharmaceutics, SSS’s Divine College of Pharmacy, Nampur Road, Satana, Nashik, Maharashtra, India – 423301
3 Department of Pharmaceutical Vhemistry, MGV’s SPH College of Pharmacy, Malegaon, Nashik, Maharashtra, India – 423203
Abstract
Dakin oxidation reaction is a widely used reaction for the synthesis of phenolic compounds such as catechol in industry, this is cheap and simple method definitely will attract attentions and find potential application in near future. A new way of synthesizing catechol was developed using the Dakin reaction in a more environmentally friendly way. In fact, aromatic aryl aldehydes can be turned into phenols at room temperature with the help of H2O2 in tap water and RO Outlet water as catalyst. It is amazing that catalytic system doesn't need activation or any transition metal catalyst, toxic ligand, additive/promoter, base, organic solvent, etc. To find out what this protocol can do, a number of substituted hydroxylated benzaldehydes were tried.
Graphical Abstract
Keywords
Main Subjects
Introduction
The idea of "green chemistry" is based on twelve guiding principles that aim to reduce or eliminate hazardous materials from the synthesis, production, and application of chemical products. As a result, the use of substances that are harmful to human health and the environment should also be minimised or eliminated [1, 2].
These principles serve as a framework for the design of new chemical products and processes. They apply to all facets of the process life-cycle, including the raw materials used, effectiveness, safety of the transformation, the toxicity, biodegradability of the products, and reagents used. Recently, they were condensed into the easier-to-remember acronym, productively [3].
To reduce the likelihood of chemical accidents, such as releases, explosions, and fires, substances and the form of a material should be employed in chemical processes [4, 5].
In the Dakin reaction, an aromatic aldehyde or ketone is converted into a phenol by being treated with alkaline hydrogen peroxide, but there must be a -OH group in the ortho- or para-position [6].
Hydrogen peroxide is one of the least expensive, most environmentally friendly, and the easiest to use oxidising agents [7, 8]. It has been utilised for a number of oxidative reactions over the years [9]. Phenols and their derivatives are widely used and serve as significant substrates in a variety of industries [10, 11]. Particularly in agrochemicals, anti-inflammatory drugs, flavourings, polymerization inhibitors, and photochemical processes [12, 13].
High temperatures were used to prepare hydrogen peroxide and sodium hydroxide in the original Dakin technique [14, 15]. Both the aryl and the acyl sp2 in the Dakin oxidation are successfully oxidised by the Baeyer-Veliger oxidation, which converts benzaldehydes into phenols, and the subsequent hydrolysis of the intermediate aryl format [16, 17].
Our research shows that even with deactivated species, the Dakin reactions may be successfully carried out when H2O2 was prepared in tap water or RO Outlet water are utilised as the catalyst system [18, 19]. This innovative H2O2 in tap water or RO Outlet water system appears to be a superior alternative to existing reagents in terms of quicker reaction times, cleaner product creation, commercial viability, and environmental sustainability when compared to the conventional reagents [20-22].
To achieve the Dakin reaction more sustainably, we have created a unique methodology [23, 24]. In reality, we can convert aromatic aryl aldehydes into phenols at room temperature by employing 30% H2O2in bore water, RO outlet water, and other prepared concentred solutions (Scheme 1).
We therefore firmly believed that this innovative and environmentally friendly approach represents an effective and highly Dakin oxidation offers an interesting alternative to the current techniques for this important synthetic conversion since it formally accomplishes synthetically difficult aromatic hydroxylation [25, 26].
Literature reviews show that WEB and WERSA are mostly made up of potassium, sodium carbonate, chloride, and a few other trace elements. In the same way that H2O2 role in chemical synthesis has grown over time, the use of this oxidant has become more common because it makes water as a by-product and has a high concentration of oxygen [27].
Because Dakin oxidation officially finishes the difficult synthetic task of aromatic hydroxylation, we were sure that this new and safe for the environment strategy were a much better way to do this important synthetic conversion than the current methods. In this study, we came up with a new, very green way to deal with this problem for this important change [28].
We have developed a novel protocol to realize Dakin reaction in a greener way. In fact, using 30% H2O2 - bore water, RO Water Outlet or 5%, 10%, 15%, and 20% K2CO3, Na2CO3, and bore water (CI) solutions, we can oxidize aromatic aryl aldehydes to phenols at room temperature.
It is amazing that catalytic system can be activated without the use of harmful ligands, additives, or promoters, transition metal catalysts, bases, organic solvents, etc. To determine the applicability of this approach, various substituted hydroxylated Benzaldehydes were tested [29].
Experimental
Materials and Methods
From Sudharshan Chemicals in Pune, India, all necessary chemicals were bought, including salicylaldehyde and hydrogen per oxide. Melting point was taken using an open glass capillary tube in a liquid paraffin bath, for the thin layer chromatographic of aluminium covered with Silica Gel 60F254 measuring 20 X 20 from the German business Merck.
Preparation of 30% H2O2 solution in high minerals containing water
In the reaction medium, we began the aerobic Dakin oxidation of several substituted hydroxylated Benzaldehydes [30]. A 30% solution of H2O2 is prepared by addition of 30% H2O2 and 70% high mineral containing such as bore water, RO, and outlet water by volume [31].
Procedure
When 3 mL of 1 equivalent of 30% H2O2 which was initially prepared in bore water, RO outlet water were mixed with salicylaldehyde at room temperature, the reaction took only 40 minutes [32].
Greener synthesis of catechol using bore water as catalyst
When the Salicylaldehyde react with 30% H2O2 (2) equivalent, catalyst 3 mL and bore water were used at room temperature, it gave catechol (Scheme 2).

Scheme 1. Previous work Vs. current work (synthesis of catechol using salicylaldehyde reacts with 30% H2O2 in tap water, RO, and outlet water

Scheme 2.The salicylaldehyde react with 30% H2O2 and bore water, it gives catechol

Scheme 3.The salicylaldehyde react with 30% H2O2, RO outlet water, it gives catechol
Greener synthesis of catechol using RO outlet water as catalyst
When the Salicylaldehyde react with 30% H2O2 (2) equivalent, catalyst 3 mL, RO outlet water were used at room temperature. It gave catechol (Scheme 3).
Greener synthesis of catechol using different prepared solutions as catalyst
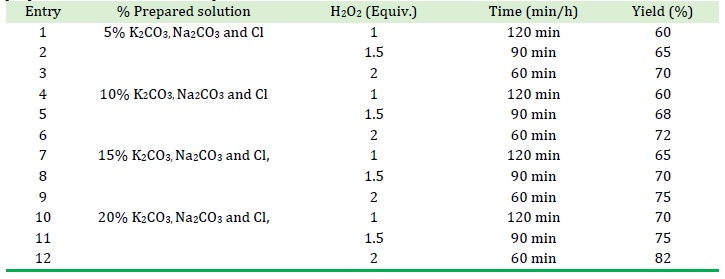
The solutions of potassium bicarbonate and sodium bicarbonate were prepared as catalyst. The adequate amount of K2CO3, Na2CO3 and bore water (Cl) were added to prepare solutions 5% K2CO3, Na2CO3 and Cl, 10% K2CO3, Na2CO3 and Cl, 15% K2CO3, Na2CO3 and Cl, 20% K2CO3, as well as Na2CO3 and Cl. The concentration solution was prepared at 5%, 10%, 15%, and 20%, when the Salicylaldehyde react with prepared solution with 30% H2O2 at room temperature. It gave catechol very shorter reaction (60 min) (Scheme 4).
Results and Discussion
Our initial efforts, which are represented in Tables 1, 2, and 3 were aimed at determining the ideal reaction conditions for our suggested process. The model reaction of salicylaldehyde with 30% H2O2 in bore water, RO outlet water, and other prepared concentred solutions at room temperature was used to carry out this synthetic approach, and it was quickly and smoothly transformed into catechol in excellent yields.
Initially, we looked at how much 30% H2O2 equivalent affected this reaction in relation to the substrate. Initial reactions were carried out employing a single equivalent of 30% H2O2 in bore water, RO outlet water, and other prepared concentred solutions at room temperature. For bore water, RO outlet water, and other prepared concentred solutions at room temperature, the reaction took place at room temperature, and after 1 hour, up to 60% of the product were isolated, respectively (Tables 1, 2, and 3).
At room temperature, the second reactions were carried out with 1.5 equivalences of 30% H2O2 in bore water, RO outlet water, and other prepared concentred solutions. After 1 hour of reaction time for bore water, RO outlet water, and other prepared concentred solutions, respectively, up to 70% of the product was isolated from the reaction, which was carried out at room temperature (Tables 1, 2, and 3).
When the amount of 30% H2O2 equated to 2, a quantitative coupling yield of 60% to 80%, respectively, for bore water, RO outlet water, and other prepared concentred solutions was attained (Tables 1, 2, and 3).
We were happy to see that under all of the settings (with various 30% H2O2 equiv.) we tried, catechol was produced in good to exceptional yields, demonstrating the viability of our suggested oxidation method (Tables 1, 2, and 3).

Scheme 4. The salicylaldehyde react with 30% H2O2 and 5, 10, 15, and 20% solutions are prepared, it gives catechol
Table 1. Effects of the amount of 30% H2O2 and time in the Dakin oxidation of salicylaldehyde in bore water at room temperature

Table 2. Effects of the amount of 30% H2O2 and time in the Dakin oxidation of salicylaldehyde in RO outlet water at room temperature

Table 3. Effects of the amount of 30% H2O2 and time in the Dakin oxidation of salicylaldehyde in different prepared solutions at room temperature

Scheme 5. Potential H2O2–WEWSA and WECMA catalysed Dakin oxidation mechanism
Table 4. Conversion of Salicylaldehye to phenols in H2O2– WEWSA and WECMA system at room temperature

This transformation is a method of choice for the straightforward manufacture of several hydroxylated phenols due to the mild reaction conditions, shorter reaction time, cost-effectiveness, operational simplicity, and extremely high yields.
A solution to this issue is presented in the shape of a fresh, gentle, and environmentally friendly replacement for this crucial transformation within the collection of prior techniques mentioned here.
There is a great deal of scientific curiosity in the process and significant acceleration for Dakin oxidation catalysed by bore water, RO outlet water, and other prepared concentred solutions, although the precise nature of active species is yet unknown. We think that the presence of alkali metal carbonates in bore water, RO outlet water, and other prepared concentred solutions, such as K2CO3 and Na2CO3, is serving as an internal base to aid the Dakin oxidations in this situation. Scheme 5 provides the most likely general mechanism for this reaction in the presence of H2O2 in bore water, RO outlet water, and other prepared concentred solutions system.
We find that these catalytic oxidations occur more quickly in bore water, RO outlet water, and other prepared concentred solutions than in traditional solvents. When compared to the previously described method for Dakin oxidation, this method uses such a gentle and environmentally friendly setting and produces such high product yields. Therefore, it appears that the current process is a practical means to convert benzaldehydes into the appropriate phenols. We also think that this is one of the best and greenest methods yet described for the title reaction in light of the results presented in this study. As a result, the community of synthetic chemistry would greatly benefit from and find the current synthetic process to be more effective.
Here, we used a greener technique to synthesize catechol with the aid of various catalystic conditions, bore water, RO outlet water, and other prepared concentred solutions. Melting point, infrared, mass, nuclear magnetic resonance, and the UV spectroscopy were used to confirm the synthesised compounds. Their % yield was also calculated (Table 4).
Conclusion
Oxalic acid dihydrate has been employed as an effective and capable catalyst with environmentally friendly character. In kinetics, it was found that ethanol had more effects on the reaction rate than methanol. It was recognized that step 1 of reaction mechanism is a rate-determining step (RDS). In step 1, dehydration process for the generation of imine-forming proceeds through the E2-elemination (concerted reaction) with a non-polar transition state that is more consistent with ethanol than methanol. Although, the reaction is enthalpy controlled in both solvent, but the non-polar transition state of step 1 has more highly ordered in relation to ethanol than methanol (ΔSǂ = -66.6 in comparison with ΔSǂ = 142.4). This is a good advantage for ethanol to provide a better environment for the reaction's progressing. The large negative value of ΔSǂ in the case of ethanol express the activated complex has a more ordered or more rigid structure in the transition state, which indicates an associative mechanism. Experimental data indicated that the second step of the reaction is a fast step. High value of activation Gibss free energy indicated that the reaction is a chemically, controlled process.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Orcid
Khemchand Rajendra Surana https://orcid.org/0000-0001-8918-1159
How to cite this manuscript: Khemchand Surana*. Tap Water and RO Outlet Water a Novel Greener Route to Catechol Synthesis in H2O2. Asian Journal of Green Chemistry, 7(3) 2023, 189-198. DOI: 10.22034/ajgc.2023.390693.1389


