Document Type : Review Article
Author
Department of Organic Chemistry Faculity of Pharmaceutical Chemistry Tehran Medical Science Islamic Azad University, Tehran, Iran
Abstract
Carbohydrazide and its derivatives are regarded as potential pharmacologically active substances. In particular, they are discussed as important anticancer, antibacterial, anti-inflammatory, anti-tuberculosis, antiviral, as well as antibacterial agents. Owing to their wide-spectrum substantial biological activity, this scaffold has called great attention to assess its skeleton synthetically and biologically. This brief review underlines the various synthetic protocols and the biological properties of carbohydrazide-containing compounds.
Graphical Abstract
Keywords
Main Subjects
Introduction
Carbohydrazides derivatives have versatile applications in particular in pharmacochemistry and material science. They would be found in structure of agrochemical compounds and different ligands to produce complexes as they are (-CONHN=CH-) moiety included.
Quinolones are described as a high importance family of synthetic drugs with convincible antimicrobial features. Therefore, they have been broadly utilized for a variety of infectious diseases [1]. The first type of synthetic quinolone included material was nalidixic acid in 1962, which has a suitable antibacterial properties against various gram-negative bacteria [2, 3]. A Quinolone, with N-1-ethyl substitution, was synthesized by locating sulfonamide moiety at 3 positions of quinolone compounds (Scheme 1). Aniline condensed with diethyl ethoxy methylene malonate to give ethyl anilinmalonate derivatives. Ethyl anilinomethylenemalonate was refluxed with diphenyl ether to give 1,4-dihydro-4 oxoquinoline-3-carboxilic acid ethyl ester derivatives, that was refluxed with ethyl iodide and potassium carbonate for 10 hours in the DMF as solvent to give substituted 1-ethyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acid ethyl ester derivatives. Refluxing with hydrazine monohydrate in absolute ethanol give 1-ethyl-4-oxo-1,4-dihydroquinoline-3-carbohydrazide derivatives. Each carbohydrazide derivative interact with p-toluenesolfonyl chloride in pyridine which act as base in dichloromethane and ethyl acetate as solvent, afforded substituted-1-ethyl-4-oxo-1,4-dihydro-3-3[1-oxo-2-hydrazino-3-[2]]quinolone derivatives. All synthesized compounds were composed of hydrazide and sulfonamide moieties at C-3 with antibacterial properties. These products tested against gram-negative E.coli, S.typhi and P.aeruginosa and gram-positive Staphylococcus aureus using disc diffusion method [1].
Diabetes and obesity are two worldwide health problems for the lack of effective drug treatment. In this work, 1,5-diaryl pyrazole derivatives were synthesized and biological activities of this products investigate and hypoglycemic activities in an in vitro model was tested. To synthesize compounds, enolates of substituted propiophenone derivatives afforded in methylcyclohexane by treatment with LiHMDS, and then with diethyl oxalate to get to tricarbonylic lithium salt, with products ranging from 60% to 97%. The cyclocondesation of phenylhydrazines and tricarbonylic compounds in solution of acid-ethanol created ethyl pyrazole-5-carboxylic acids by potassium hydroxide at 50 °C from 50% to 87% yield. In the end, object product was produced by formation of the acyl chloride derivatives from pyrazole 5-carboxylic acids and treatment with aminopiperidine and DIPEA in chloroform (Scheme 2) [2]. Compounds were obtained from changing R group evaluated in vivo hypoglecymic activity STZ-nicitinamide rat model of diabetes. The anti-diabetic activity of compounds was tested at 50 mg/kg of an oral dose, using glibenclamide for positive controlling.
Hydrazides use in the structure of some heterocycles with biological activities. The common reaction for synthesis of chromene containing hydrazide is the reaction of indoline-2,3-diones with suitable hydrazide. Compounds which have chromene in their structures show anti-cancer activities by inhibition against aromatase, carbonic anhydrase, and steroid sulfatase enzymes. Indoline-2, 3-diones has anticancer activities by suppressing tyrosine kinase or caspase.

Scheme 1. Synthesis of carbohydrazide 4a and sulfonamide 5a derivatives

Scheme 2. (i) LiHMDS, diethyl oxalate, methylcyclohexan, reflux 17 h, (ii) substituted arylhydrazines, EtOH, H2SO4, reflux, 6-8 h, (iii) KOH, EtOH, 50 °C, 12 h, (iv) SOCl2, PhMe, reflux, 2 h, and (v) 1-aminopiperidie, DIPEA, CHCl3, 0 °C to r.t., 4 h

Scheme 3. Synthesis of hydrazones 3a-i
Route for the synthesis of hydrazones containing chromene is the hydrazide reaction with indoline-2,3-diones (Scheme 3). Chromene-2-ones’s ring has opened in mild condition because pyran-2-one ring of chromene is very reactive. Ring opening at the lactone acyl center in nucleophilic conjugate addition to the carbon-carbon double bond. Chromene-based hydrazones synthesis started with ethyl 3-hydrazinyl-3-oxopropanoate (3b). Accordingly, suitable indoline-2,3-dione derivative treatment with (3a-g) in refluxing ethanol led to ethyl 3-oxo-92-92-oxoindolin-3-ylidene)hydazinyl) propanoates. Hydrazones with suitable salicylaldehyde condensed in piperidine resulted 2-oxo-N’-(2-oxoindolin-3-ylidine)-2H-chromene-3-carbohydrazides [3].
Progress in antiviral producing for influenza has considerable importance. Four antiviral synthetic drugs are available. However, influenza viruses evolve resistance to available antivirals. A carbohydrazide compound increased the thermal stability of effector domain, is 3-hydroxy-N-[(Z)-1-(5,6,7,8-tetrahydronaphtalene-2-yl)ethylidenamino]naphthalene-2-carboxyamide (HENC). Antiviral activity of HENC is determined, HENC inhibits virus activity. Such carbohydrazide compounds, similar to HENC, have not been investigated inhibitory actions to influenza virus. HENC analogs are not available and should synthesize. Syntheses of HENC analogs involve the esterification of 2-hydroxy 3-naphtaoic acid with ethanol and H2SO4 as a catalyst in refluxing mode for 8 hours (Scheme 4). Produced ester was converted into the hydrazide with hydrazine in refluxing mode in the ethanol for 8 hours. The hydrazide with previously produced ketone condensed to form ketonehydrazide compound. Ketone was produced by Fridel Crafts acylation [4].
Semicabazides derived from carbohydrazides obtained from isocyanate reaction as a starting material for heterocycle compounds like as 1,3,4-oxadiazoles and 1,2,4-triazole-3-ones (Scheme 5a).

Scheme 4. Synthesis of HENC and analogs
Carbohydrazide derivatives determined as biologically active compounds, their catalytic activity seldom reported. However, some derivatives could be ligands for asymmetric synthesis. Aziridine are applied as catalyst in asymmetric synthesis. Chiral ligands containing aziridine were used as catalyst in different asymmetric reactions. Chiral alcohols containing aziridine is efficient catalyst for asymmetric diethyl- and phenylethynyzinc addition to different aliphatic and aromatic aldehydes and the addition of diethylzinc to α, β-unsaturated carbonyl compounds (Scheme 5b).
Acid hydrazides synthesized by reaction of esters with hydrazine hydrate. Methyl-(S)-N-triphrnylmethyl aziridinate transformed into (S)-N-triphenylmeyhyl aziridinehydrazide (Scheme 5c) [5].
Several drug molecules with antiviral properties show harmful effects, for example, penicillin cause hypersensitivity and tetracycline liver damage. Formazans have important uses in medical field as antiviral, anti-inflammatory, anti-tubercular, anti-Parkinson, anticancer, and anti-HIV. Formazans skeleton (-N=N-C=N-NH-) named as azohydrazone group, that is a good carrier for π-bonding and has chelating activities. Formazans applied as dyes, complex formation reaction as ligand and indicators for redox reactions. Crown formazan derivatives are applied as carriers for cesium ion selective electrodes and determination of lithium by spectrophotometry. Formazans derivatives synthesized from aniline diazonium salt by Schiff bases of 3,4-dimethyl-1H-pyrrole-2-carbohydrazide with antimicrobial and antioxidant propertyes [6] (Scheme 6).

Scheme 5. a) Synthesis of semicabazides, b) asymmetric addition of diethyl- and phenylethynylzinc to benzaldehyde, and c) synthesis of (S)-N-triphenylmethylaziridine hydrazide 5ii

Scheme 6. Synthesis of Formazans
Due to the biological properties of ligands and metal complexes of thiocarbazide, carbohydrazide, semicarbazide, and Schiff base derivative increase effort in this field. Most attention was paid to the semicarbazide and thiocarbazide Schiff base derivatives due to much more biologically activities compare to much less carbohydrazide Schiff base ligands. Schiff base derivatives of thiocarbazide ligands are much more studied compare to the corresponding oxygen analog carbohydrazides. Polydentate ligand system interested due to the coordination properties rise from keto and enol tautomeric equilibrium similar to hydrazones. Most carbohydrazide complexes derived from Schiff base ligands exhibit enol form. Schiff base ligand with H3bsc[1,5-bis(salicylidene) carbohydrazide)] have aptitude to attach two metal ions in the enol form while complex forming. In this work, Ni(ClO4)2.6H2O in acetonitrile at room temperature treatment with H3bsc ligand to produces the mononuclear complex in keto form of ligand [2]. All three acidic groups of free ligand were protonated in result complex is neutral. Due to the electronegative oxygen atom in this kind of ligands system prefer to coordinate in the enol form. Treatment of [Ni2(µ-OH2)(O2CCMe3)4(HO2CCMe3)4] with H3bsc in methanol produce the tetranuclear complex [Ni4(0.5bsc)2(O2CCMe3)3(CH3OH)4](2).H2O.2CH3OH. The ligand coordinates to three Ni(II) centers (Scheme 7) [7].
Tuberculosis is one of medicinal chemist challenging because resistance to be existence drugs. Pyrazine derivatives have anti-tubercular property. Pyrazinic acid hydrazides (Scheme 8a) are in opposition Mycobacterium tuberculosis produced from condensation of aromatic aldehydes. Synthesis of methyl pyrazine carbohydrazide derivatives have a three-step procedure, firstly 5-methylpyrazinoic acid cooperative with 5-methylpyrazinoate in the presence of ethanol and Conc.H2SO4 as a catalyst. 5-methylethylpyrazinoate transform to 5-methylpyrazinoic acid hydrazide by hydrazine hydrate. Finally, the 5-methylpyrazinoic acid hydrazide was condensed with various substituted aromatic aldehyde in ethanol environment and produced different substituted phenylmethylidene-5-methlpyrazine-2-carbohydrazide derivatives (Scheme 8b) [8].

Scheme 7. Coordination behavior of the ligand in 1, 2, and 3

Scheme 8. a) Pyrazine derivative with anti-tubercular activities, and b) 5-methylpyrazine-2-carbohydrazide derivatives
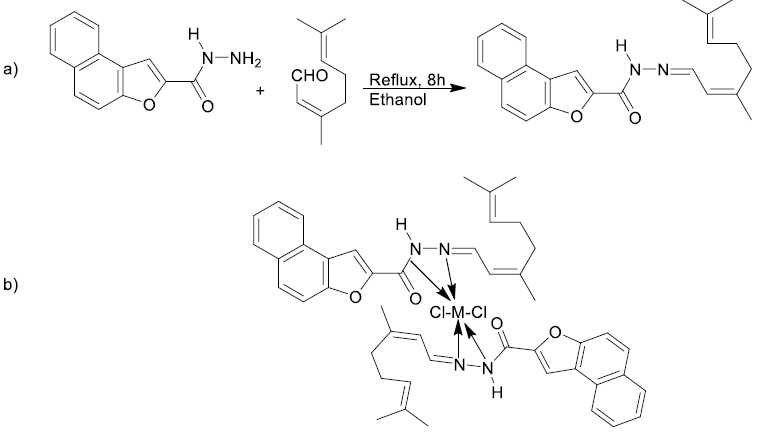
Naphthofuran has biological activity found in many important natural products. (±)-Laevigatin, (+)-heritol and balsaminone are natural naphthofurans that have pharmacological and cytotoxic activities. Naphthofuran condensed with different heterocycles show a wide range of activities. This heterocycles and their metal complexes have antioxidant property. Schiff base complexes of this compound possess antifungal, antitumor, and antibacterial activities. Metal when coordinated to molecule with biological molecules improve and increase their activity. To synthesize the products of naphthofuran-2-carbohydrazide and citral mixed in hot ethanol boiled in reflux condition for 8 hours on a water bath until fade yellowish solid separated (Scheme 9a). Then, obtained products mixed with metal salts [Cu(II), Co(II), Ni(II) ,Cd(II), Hg(II), and Zn(II) in reflux condition with ethanolic solution to produce complexes (Scheme 9b). For adjusting PH to 6.0-7.0, sodium acetate was used [9].
Complexes of dimedone and carbohydrazide prepared in methanol medium is [M(TML)X}X2 form; that TML is a macrocyclic ligand; M = Cr(III), Fe(III); X = Cl−, NO3−, and CH3COO−. The macrocyclic complexes are used as metal radiotherapeutic agents and extractants. If metal is lanthanides e.g., Gd3+ are used in MRI as contrast agents. The macrocyclic complexes with transition metal have biological activities e.g., antifertile, antiviral, anticarcinogenic, antifungal, and antibacterial. Macrocyclic complexes of Cr(III) and Fe(III) with ligand which produced from dimedone and carbohydrazide. Ligand synthesized by condensation of dimedone and carbohydrazide with trivalent metal salts. Cr(III) and Fe(III) salt added to hot and stirred methanolic solution of carbohydrazide, this solution was refluxed for 0.5 h. Methanolic solution of dimedone added to refluxing mixture, and then the mixture was refluxed for 6-8 h (Scheme 10) [10].
Scheme 9. a) Synthesis of Schiff’s base ligand, and b) structure of Zn(II), Co(II), Cd(II), Ni(II), and Hg(II) complexes

Scheme 10. Synthesis of macrocyclic complexes with trivalent metal salts
New series of carbohydrazide derivatives β-isatin aldehyde was synthesized by condensation reaction of thiocarbohydrazide or carbohydrazide with 5-subistitued isatin. Products are active via different stretch of DNA and RNA viruses. Isatin derivatives have a long range of properties like antimalarial, cytotoxic, antibacterial, antiviral, and antifungal. Methisazone (N-methylisatin-β-thiosemicarbazone) is effective via vaccinina and smallpox. Isatin derivative e.g., N,N’-di substituted thiosemicarbazone were inhibitory against HIV-1 and N-methylisatin-β-thiosemicarbazone was effective against infection of encephalitis virus. To synthesize monothiocarbohydrazones, mixture of substituted aldehyde and thiocarbohydrazide in ethanolic solution with a few drops of glacial acetic acid refluxed for 1 h. For synthesis of β-isatin aldehyde-N,N’-thiocarbohydrazones, in solution of monothiocarbohydrazone and substituted isatin in ethanol medium added glacial acetic acid to start reaction. For synthesis of bis-isatin derivatives, the mixture of thiocarbarbohydrazide or carbohydrazide was blended with suitable isatin derivatives in ethanol and acetic acid, and then the mixture was refluxed for 2 hours (Scheme 11) [11].
Tri-substituted 1,3,5-triazine arrangement is important in medicinal chemistry. These compounds have biologically activities e.g., antimalarial, anticancer, anti-metastatic, anti-gastric, and antimicrobial. 4-amino-6-(arylamino)-N-phenyl-1,3,5-triazine-2-carbohydrazide synthesized in 2 steps using arylbiguanide hydrochloride salts produced from substituted aniline and dicyandiamide. Arylbiguanide salts neutralized by a mixture of sodium methoxide and methanol and react with dimethyloxalate in methanol by refluxing method. This reaction yielded intermediate product which isolated and recrystallized, and then react with phenyhydrazine in ethanol medium by refluxing method in the presence of glacial acetic acid as catalyst (Scheme 12a). 4-amino-6-(arylamino)-N-benzyl-1,3,5-triazine-2-carboamides synthesized similar procedure. In this reaction, arylbiguanide cyclized with diethloalate to produce triazine ethyl ester intermediate in refluxing method. Intermediate react with benzylamines in dioxane solvent by refluxing and presence of acetic acid as catalyst (Scheme 12b) [12].
Botanical pesticides were extracted from plants that have not environment residues are attractive alternatives in green pesticides. However, they have active component east to decompose. Based on this reason, active substances from plant origin are used to develop novel effective pesticides. β-Carboline alkaloids are attractive to pharmaceutical chemists found in nature. Tetrahydro-β-carboline-3-carbohydrazide 13 one of β-carboline derivatives which is active against tobacco mosaic virus (TMV).

Scheme 11. Synthesis of bio-istatin derivatives

Scheme 12. a) Synthesis of 4-amino-6-(arylamino)-N-phenyl-1,3,5-triazine-2-carbohydrazides Reagent: (i) NaOCH3, CH3OH, reflux, 3 h, (ii) dimethyoxalate, CH3OH,reflux, 4 h, and (iii) phenylhydrazine, AcOH, EtOH, reflux, 12 h, and b) synthesis of 4-amino-6-(arylamino)-N-nenzyl-1,3,5-triazine-2-carboxamides
Acylhydrazone compounds are biologically active due to containing active portion (-CONHN=CH-) extensively exist in drug compounds as insecticide and pesticides e.g., pymetrozine, metaflumizone, and diflufenzopyr. Using 13 compound new series of tetrahydo-β-carboline derivatives synthesized. The schematic of synthesis routes are shown in Scheme 13 [13].
New triazine series e.g., 4-amino-6(arylamino)1.3.5.-triazine-2-carbohydrazide and N'-phenyl-4,6-bis(aryl-amino)-1,3,5-triazine-2-carbohydrazides have anticancer properties. 4-amino-6-(arylamino)-1,3,5-triazine-2-carbohydrazide was synthesized in two steps from aryl biguanide hydrochloride salts produced from substituted aniline and dicyandiamide. Arylbiguanide hydrochloride was neutralized by sodium methoxide/methanol mixture in treatment with dimethyloxalate in reflux in refluxing methanol. This reaction gave methyl 4-amino-6-(arylamino)-1,3,5-triazine-2carboxylates as intermediate. Intermediat react with hydrazinehydrate by refluxing mode in ethanol to generate new triazines. N'-phenyl-4,6-bis(arylamino)-1,3,5-triazine-2-carbohydrazides produce in similar method from bisarylbiguanidin hydrochloride salts produced from sodium dicyanamide and substituted aniline. Salts was neutralized by mixture of sodium methoxide/methanol in refluxing methanol and treatment with dimethyloxalate to generate methyl 4,6-bis(arylamino)-1,3,5-triazine-2-carboxylates as intermediate. Intermediate reacts with phenylhydrazine in refluxing ethanol in presence of glacial acetic acid as catalyst (Scheme 14) [14].
Benzimidazole is essential structure for the development of medicinal compound due to its derivatives have pharmaceutical application e.g., strong inhibitor against GSK-3β(glycogen synthase kinase). The carbohydrazide motif has suitable H-bond donor property for interact powerfully with GSK-3β site and consequently give greater GSK-3β inhibition. Conjugation of benzimidazole with aryl/heteroaryl carbohydrazides and 2-(alkyl/aryl)amino-1,3,4-thiadiazoles via methylene linkage accomplished. Synthesis of bezimidazole derivatives with carbohydrazides and 1,3,4-thiadiazoles shown in Scheme 15.
The main intermediate used for synthesis was 2-(2-methyl-1H-benzimidazol-1-yl)acetohydrazide produced from treatment of 2-methyl-1H-benzimidazole with ethyl bromoacetate using anhydrous potassium carbonate just as a base, succeed by the reaction with hydrazine hydrate. The benzimidazole thiosemicarbohydrazides were produced by condensing compound 15b with suitable isothiocyanates. Compound 15c was cyclized by cond.H2SO4 at room temperature to give benzimidazole-1,3,4-thiadiazoles (Scheme 15) [15].

Scheme 13. Synthesis of compound 13

Scheme 14. Synthesis of 14(a-e)1-3 compounds. Reagents: (i) NaOCH3, CH3OH, room temp, 3 h, (ii) dimethyloxalate, CH3OH, reflux, 4h, and (iii) hydrazine hydrate, EtOH, reflux, 3 h
The protozoa Trpanosoma cruzi causes Chagas disease. Blood-sucking triatomine vectors (kissing bug) transmitted parasite to human. This illness exposed two clinical states: acute and chronic. The acute contagion is commonly asymptomatic, but chronic contagion has been companioned with high amount of morbidness and death. Nitroheterocyclic drugs e.g., benznidazole and nifurtimox have effect against this contagion. Both drugs are effective against acute contagions, but due to disagreeable side effects about use for chronic patients are disputed, besides their effect on illness remedy is low. Benznidazole and nifurtimox are not ideal drugs due to high side effects and low effects, the search for new effective drugs contra T.cruzi, with low toxicities and expanded efficiencies during the chronic state. Effect of Benznidazole and nifurtimox contra trypanocidal depends on the reactive metabolites formation that is mediate for parasite killing. Benznidazole reduction with type I nitroreduction produced glyoxal, widely known toxic metabolite. Nifurtimox acts by reduction of nitro group to unsteady nitroanion radical, that to generate so high toxic oxygen reduced metabolites (hydrogen peroxide, superoxide anion, and hydroxyl radical). Unfortunately, none of drugs are not so active in the chronic sate of the disease. Derivatives of benzimidazole have activity contra T.cruzi. Some derivatives cytotoxicity was tested on mouse macrophage cell. Synthesis of compound has shown on Scheme 16 [16].
b-Glucuronidase is a glycosidase which catalysis b-linked glucuronides to produce different derivatives and free glucuronic acid. b-Glucuronidase has been separated from many organism e.g., bacteria, plants, humans, and animals. One of application of glucuronidase, has been used in structural investigations of proteoglycans and diagnostic research laboratories. Quinoline is an aza-heterocyclic compound and like other heterocycle compounds have diverse biological activities. Quinoline is a tertiary base which can endure both electrophilic and nucleophilic substitution reactions. Quinoline has biological properties and is found in several pharmacologically compounds.

Scheme 15. Synthesis of benzimidazole derivatives from carbohydrazides and 1,3,4-thiodiazoles
Moiety of quinoloine is not toxic to human and its derivatives have antibacterial, antimalarial, anti-HIV, antiprotozoal, antifungal, and anticancer activities. Quinoline derivatives have inhibitory potential against b-Glucuronidase. N-aryllidenequinoline-3-carbohydrazides derivatives were synthesized via the reaction of 8-fluoro-4-hydroxy-3-quinoline carbohydrazide with suitable aldehyde in methanol solvent in the presence of glacial acetic acid as catalyst. 8-Fluro-4-hydroxy-3-quinoline carbohydrazide is essential intermediate for the synthesis final compounds. One step reaction of ester in methanol is 8-flouro-4-hydraoxy-3-quinoline carboxylate with hydrazine hydrate in excess amount (Scheme 17) [17].

Scheme 16. Reagents: (a) CH3COOH, HCl, reflux, 8 h (b) i) CDI, CH3CN, ii) tert-butyl carbazate, 40-50 °C, 10 h, (c) TFA, reflux, 5 h; (d) iPrOH, aromatic aldehydes, 50-70 °C, 10 h

Scheme 17. Synthesis of N-arylidenequinoline-3-carbohydrazide
1,5-Diarylpyrazole derivatives such as rimonant 18a have in vivo hypoglycemic effect by reason of its interaction with incidental cannabinoid receptor 1 (CB1R) which is antagonist/inverse agonist for treatment of metabolic syndrome and obesity. Other derivatives of 1,5-diarylpyrazole 18b shows in vivo hypoglycemic and in vivo antioxidant activities. Compound 18b have a vanillinic hydrazone portion which is responsible for scavenging radicals’ properties and can be alter for other antioxidant parts (Scheme 18).

Scheme 18. Structure of the pyrazole derivatives rimonabant 18a and the lead 18b
To synthesize compounds, firstly 4-chloropropiophenone was reacted with LIHMDS in methylcyclohexane, and then treated with diethyl oxalate to produce the tricarbonylic compound. Condensation of 17d with 3,4-dichlorophenylhydrazine in sulfuric acid and ethanol generate pyrazole ring, that a basic hydrolysis produced the pyrazole-3-carboxylic acid 17e. Stirring of 17e yield in SOCl2/toluene and the next reaction with tert-butyl carbazate, generated N'-Boc carbohyrazide 17f. BOC group separated with trifluoroacetic acid and condensation with suitable aromatic aldehyde produced the hydrazonic yields (Scheme 19) [18].
Cardiovusvascular diseases cause over 17 millions deaths around the world each year. The essential reason of most cardiovascular diseases is atherosclerosis. Platelets are take part with atherosclerosis because the ability to be the injured blood vessel wall and expand the procoagulant reply. Pyrazolopyridine derivatives have ability to inhibitory of platelet aggregation potential induced by arachidonic acid and collagen. Interconversion of functional group from ester 21a and 21b to lead to carbohydrazide with hydrazine monohydrate in reflux mode. A mixture of carbohydrazide derivatives and absolute ethanol was added to aromatic aldehyde in the presence of 5 drops of HCl. The mixture refluxed and stirred to produce yield (Scheme 20) [21].
Monoamine oxidases (MAO) are flavoprotein. MAO has the catalytic activity due to Flavin adenine dinucleotide FAD) co-factor which bound to a cysteine amino acid covalently. MAO catalyzes deamination by oxidation of vasoactive and neuroactive the oxidative deamination in the brain. MAO activity related to anxiety, depression, and Parkinson’s disease. Inhibitors of monoamine oxidase are used to lead an increase in the level of neurotransmitters and so play efficient role therapeutic cure via psychiatric and neurological diseases like Parkinson’s. 1,2-Benzothiazines are heterocyclic compound with different pharmacological applications e.g., isoxicam, sudoxicam, piroxicam, and meloxicam and others. Benzothiazine derivatives produced from condensation with carbohydrazide.

Scheme 19. Synthesis of compounds 18g(1-10): (a) LiHMDS, diethyl oxalate. MCH, reflux, 17 h, (b) 3,4-dichlorophenyl hydrazine, EtOH, H2SO4, reflux, 8 h; (c) KOH,EtOH. 50 °C,12 h, (d) SOCl2, toluene, reflux, 2 h, (e) tert-butyl carbazate, DIPEA, CHCl3, 0 °C, reflux,4 h, and (f) TFA, the vanillin, AcOHcat, CHCl3, reflux

Scheme 20. Synthesis of N’-benzylidene-carbohydrazde-1H-pyrazolo[3,4-b]pyridine derivatives; (i): N2H4, EtOH and (ii) aromatic aldehyde, EtOH, HCl
4-Hydroxy-N’-[benzylidene/1-phenlethlidene 1,1-dioxides series were synthesized sodium saccharin that was N-alkylated with methyl chloroacetate. Gabriel-Colman rearrangement of N-alkylated saccharin generated methyl 4-hydroxy-2H-1,2-benzothiazine-3-carboxylate dioxide. It was produced high yield of ester when the reaction was done under nitrogen and lack of moisture. Synthesis of derivatives was shown schematically in Scheme 21 [22].

Scheme 21. Synthesis of 4-hydroxy-N’-[benzylidene or 1-phenylethyllidene]-2-h/methyl/benzyl- 1,2-benzothiazine-3-carbohydrazide 1,1-dioxides.
Concusion
Carbohydrazides display a significant pharmacophore with numerous pharmacological properties, and some carbohydrazide containing derivatives have been employed as pharmaceutical agents. This short review demonstrates that carbohydrazide derivatives are potential biologically active ingredients and, hence, their design and preparation is an interesting field of research. Based on several literature survey carbohydrazide derivatives reveal different biological activities including anticancer, anti-inflammatory, antiviral, antibacterial and anti-tubercular etc. the possible enhancement in their properties can be further obtained by modification of the substituents on the carbohydrazide nucleus. It is anticipated that this brief review will be useful in the next studies and for suggesting novel approaches for producing more potent carbohydrazide containing compounds.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Orcid
Somayeh Sattari Alamdar
https://orcid.org/0000-0002-0354-7453
How to cite this manuscript: Somayeh Sattari Alamdar. A Review on Synthesis of carbohydrazide derivatives. Asian Journal of Green Chemistry, 7(2) 2023, 91-109. DOI: 10.22034/ajgc.2023.383255.1370


