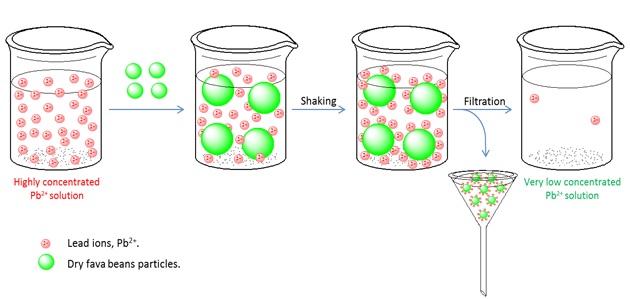
Document Type : Original Research Article
Authors
- Salaheddin A. Sharif 1
- Hameda Ali Mohamed Naser El-Moghrabi 2
- Widad Saed El-Mugrbi 2
- Aya Ibrahim Alhddad 3
1 Department of Chemistry, Faculty of Arts and Science, University of Benghazi, Ghemines, Libya
2 Department of Botany, Faculty of Arts and Science, University of Benghazi, Ghemines, Libya
3 Department of Biology, Faculty of Education, University of Benghazi, Ghemines, Libya
Abstract
Environmental pollution caused by toxic contaminants specially heavy metals is a remarkable issue which directly related to all human activities and mainly has negative effects on human health. Bioremediation is one of the reliable green techniques used for the removal of contaminants form ecosystems. In particular, phytoremediation method is considered as one of the potential approaches for the removal of heavy metals such as the toxic lead metal from environment. We have designed a simple experiment utilized for the removal of lead metal from its solutions. In this phytosorption (biosorption) technique, a dried fava beans powder (Vicia faba L.) was used to take up the lead ions (Pb2+) from their solutions. It was found that the fava beans biomass have removed up to 96.6% of the lead ions from lead nitrate solutions prepared in different concentrations.
Graphical Abstract
Keywords
Main Subjects
Introduction
Environmental pollution, which found in all earth ecosystems; soil or land, water, and air, has become a serious concern to all human sections in particular public health [1-4]. It can be raised from natural sources and anthropogenic activities such as agriculture, industry, and mining. Toxic heavy metals represent the most common and environmentally pollutants especially in agricultural areas [5-12]. Toxicity of heavy metals refers to many factors such as the type of heavy metal, chemical structure, dosage, duration, and method of exposure of organisms to heavy metal. Furthermore, solubility of heavy metals in water even at low concentrations, ability to accumulate in cells, biomagnification through food chain, resistance to biological and chemical degradation, or breaking down into non-toxic forms, and difficulty for elimination by chemical or biological transformation are the other features of heavy metal toxicity [10, 11]. Removal of toxic heavy metals form ecosystems requires many governmental and community efforts. Solutions for this issue lie in two patterns: (1) prevention or reducing industrial, domestic, and agricultural effluents of heavy metals in ecosystems, and (2) removal of heavy metals from contaminated environment by suitable treatment processes; which known as decontamination or remediation processes [12, 13].
The bioremediation processes are the most common and green innovative methods for the removal of heavy metal from the contaminated environmental systems. Bioremediation is an innovative, environmentally friendly, and promising technology that uses biological organisms (plants, animals, and microorganism) for purifying the polluted ecological systems by removing, degrading, and/or recovery of a wide range of organic and inorganic (heavy metals) pollutants [1, 3, 4, 5]. These benign techniques have showed high efficiency and low cost compared to the traditional ones. The principles of these techniques depend on the high affinity of biological compounds in plants, animals, and microorganisms for the metal ions for the formation of stable organometallic compounds that can be then removed [13]. The bioremediation process by plants is known as phytoremediation (plant-based clean-up or plant-based environmental remediation). On the other hand, it is called zooremediation
for animals, whereas it is named microbial remediation for microorganisms. These technologies are usually low-cost, environmentally friendly, and sustainable methods [1, 3, 4, 5].
Generally, the phytoremediation can be defined as a new, innovative, cost-effective, efficient, sustainable, and green technology that uses either natural or genetically-modified plant species to clean up and detoxify the contaminated ecosystems from various organic and inorganic hazardous pollutants, toxins, and wastes to improve environment and make it more sustainable or to render the contaminants harmless [1, 2, 3, 5, 11]. Phytoremediation concept can be divided into six mechanisms or technologies, depending on the type of pollutant, process, and applicability; (1) phytoextraction, (2) phytodegradation, (3) phytofiltration or rhizofiltration, (4) phytostabilisation, (5) phytovolatilisation, and (6) rhizodegradation, or Phytostimulation[1, 3-5]. The phytoextraction is also named phytoaccumulation, phytoabsorption, or phytosequestration. The phytoextraction process represents the use of plants to uptake toxic heavy metal contaminants from a soil matrix, mobilize, and accumulate them in aboveground tissues, and then remove them [1, 3-5]. Phytoremediation technology has used several plants as bioaccumulators or biosorbents for the removal of heavy metals from contaminated soil and water areas. These plants response to or interact with heavy metals in different mechanisms. There are many metal-biosorption mechanisms that control the metal hyperaccumulation in plant cells. These mechanisms can be understood by physiological, biochemical, and molecular explanations. The main principle of these mechanisms is to take up metal from ecosystem using ion-transport proteins by chelation and translocating metal ion to specific plant tissues or parts. These mechanisms were dependent on the type of plant cells and metal ions. These biological interaction processes between cell proteins and metal ions are; (1) bioaccumulation process, (2) biosorption process, and (3) bioreduction process [4, 5]. In the biosorption approach, there are different mechanisms and chelation of metal ions with biological molecules such as proteins through the formation of complexes is one of the most common ones. In the phytosorption process, the dead biomass of plant was used to uptake metal ions from the aqueous solutions. This technique showed high efficiency for the removal of heavy metals from industrial wastewater [5]. According to the high potential toxicity of lead metal, many governments have banned the use of many lead metal products [14-16]. Phytoremediation of lead-contaminated aqueous solutions and aquatic ecosystems using plant-based biomass as biosorbents has an attractive approach for researchers [16, 17]. For example, fava beans were examined for the removal of lead, cadmium, and zinc from wastewater [18].
In this study, the aim is to assess the potential of bean-biomass for the removal of the toxic lead heavy metal ions (Pb2+) from their aqueous solutions. The developed approach can be used to utilize the beans plant as bioindicators for environmental pollution and as scavengers for the toxic heavy metals from aquatic systems.
Experimental
Materials and methods
Heavy metal solution samples were prepared in different concentrations (50, 100, 200, 500, and 1000 ppm) from dissolving lead(II) nitrate salt, Pb(NO3)2, in distilled water and used in experiments.
Biomass
The biomass of plant Vicia faba L. (fava beans) was chosen from the local markets and grinded in a powder form to be used as an absorbent for the removal of lead ions from its aqueous solutions.
Experiment and sample analysis
A 10 g of plant biomass was added to each heavy metal solution concentration separately in a container, and then they were shaken well by hand at room temperature for 30 minutes. After settlement of biomass solutions (18-36 hrs), they were filtered through filter paper (Quality Type: GF) twice. The filtered solutions were stored in the lab refrigerator for 3 months. The amount of remaining heavy metal was then determined using the flame atomic absorption spectroscopic (FAAS) instrument (Perkin Elemer 500).
Results and Discussion
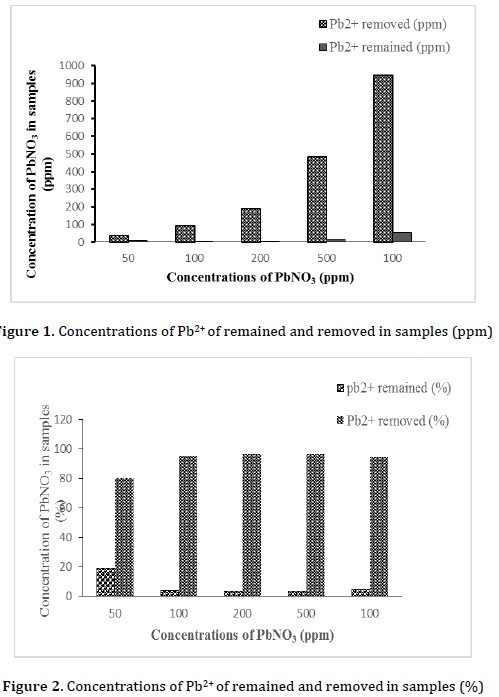
All lead metal concentrations of samples were detected using flame atomic absorption spectroscopic (FAAs) instrument at room temperature of 24.3 °C and a pH of solutions at 3.79-5.11 (Table 1) and (Figures 1 and 2). It was found that lead ion concentration levels were significantly decreased due to the absorption of fava bean biomass via chelation between bean-protein and lead ions. For the sample 1, which had a concentration of lead ion with 50 ppm before bean-biosorption process, it was found that Pb-concentration decreased to 9.705 ppm (19.4%) with heavy metal removal of a 40.295 ppm (80.6%). This sample showed the least efficient removal of metal ion within all sample. On the other hand, the samples 2, 3, 4, and 5 gave a remaining lead concentration with 4.287, 6.987, 17.10, and 52.77 ppm (4.3, 3.5, 3.4, and 5.3%) with heavy metal uptake with 95.713, 193.013, 482.90, and 947.23 (95.7, 96.5, 96.6, and 94.7%), respectively. Mainly, the best biosorption process was referred to the sample 4 with a 96.6% removal of heavy metal.
These results were inconsistent with the previously reported study that showed the potential of Vicia faba L. as biosorbent for the removal of some heavy metals from their aqueous solutions [18].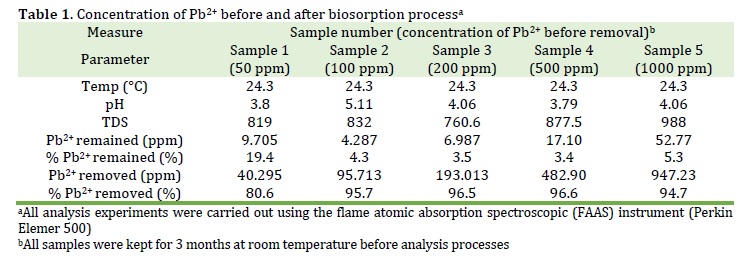
Conclusion
Contamination of ecosystems by pollutants such as heavy metals consists a significant concern and became a real threat to human health. Bioremediation processes are potential green methods for cleaning polluted environments. Phytoremediation technique is one of the most efficient processes for removal of heavy metals from different ecosystems. The resulting data from experiments that we developed showed that dried fava beans biomass can be used as an excellent biosorbent for the removal of toxic lead (Pb2+) heavy metal from its aqueous solutions at low concentrations. Due to the high affinity of fava beans-protein to the metal ions, this methodology can be applied in the contaminated aquatic environmental systems with both toxic and non-toxic heavy metals.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Orcid
Salaheddin A. Sharif
https://orcid.org/0000-0002-0247-3333
Hameda Ali Mohamed Naser El-Moghrabi
https://orcid.org/0009-0001-3349-1454
Widad Saed El-Mugrbi
https://orcid.org/0009-0003-9311-0312
Aya Ibrahim Alhddad
https://orcid.org/0009-0009-0068-301X
How to cite this manuscript: Salaheddin A. Sharif*, Hameda Ali Mohamed Naser El-Moghrabi, Widad Saed El-Mugrbi, Aya Ibrahim Alhddad. Fava beans (Vicia faba L.) phytosorption of Pb2+ ions from its aqueous solutions. Asian Journal of Green Chemistry, 7(1) 2023, 85-90. DOI: 10.22034/ajgc.2023.385586.1372