Document Type : Original Research Article
Authors
Department of Chemistry, Payame Noor University, P.O. Box 19395-4697, Tehran, Iran
Abstract
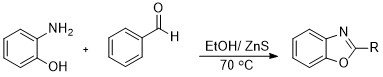
A simple efficient and eco-friendly method has been developed for the synthesis of benzoxazole derivatives by one-pot two-component reaction of o-aminophenol and various aldehydes catalytic by nanoparticles Zn-S use ethanol has been described as solvent at 70 °C. The present method has several advantages such as high yields, easy purification, mild reaction conditions, easy work-up, and short reaction times. Likewise, Zn-S nanoparticles are a catalyst that is easily synthesized, cheap, stable in air and humidity, heterogeneous, and green.
Graphical Abstract
Keywords
Main Subjects
Introduction
Benzoxazole moieties the key structures feature of a large number of biologically active natural products and drug discovery [1]. In recent years, the synthesis of these compounds has attracted the attention of many researchers, because many of these derivatives show different spectrums of medicinal activities, including anti-tumour [2], and anti-viral [3], anti-microbial [4] DNA inhibitors [5], anti-cancer [6], and anti-biotic [7]. In recent years, the condensation reaction of o-aminophenol with various aldehydes [13] in the presence of catalysts such as Ga(OTf)3 [8], o-Benzenedisulfonimide [9], Ni-SiO2 [10], PEG-SO3H [11], and CuO [12] has been reported.
Recently, ZnS is an important member of this family and it has been extensively investigated [13]. Nano Zn-S has attracted a tremendous amount of attention because it's remarkable properties such as low cost, easy of synthesis, high stability, and small size [14].
Due to the importance of benzoxazole and catalytic ability of zinc nanosulfide in organic reactions, we would like to report a new method for the benzoxazole synthesis in the presence of catalytic amounts of zinc nanosulfide at 70 °C and ethanol solvent.
Experimental
Materials and methods
All materials were purchased from Fluka, Aldrich and Merck and used without further purification. The products were characterized by comparing their physical properties and spectroscopic data with those reported in the literature. Infrared (IR) spectra were recorded on KBr Pellets on a Shimadzu IR Presting-21 spectrophotometer in the range of 4000–400 cm−1. NMR spectra were recorded in CDCl3 on a Bruker Advanced DPX 400 MHz spectrometer using TMS as an internal reference. Melting points were obtained in open capillary tubes and were measured on a Buchi melting point B-540 B.V.CHI apparatus.
Synthesis of nano particles sulphid zinc
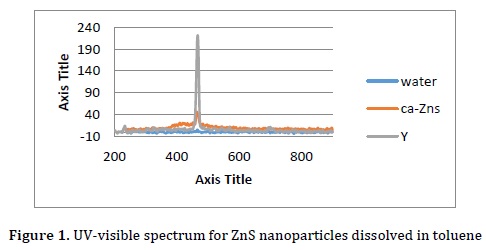
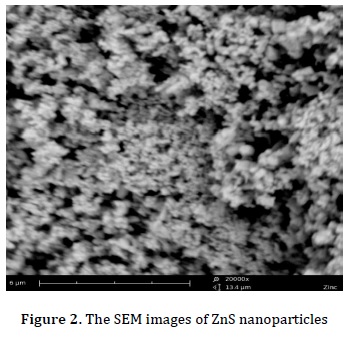
First, 0.25 g of carboxymethylcellulose was dissolved in water, and then 0.05 M is prepared from the solution of ZnCl2 and Na2S. Next, ZnCl2 solution was added dropwise under stirring at room temperature after 30 min. The solution of Na2S nanoparticles was filtered with filter paper, and then dried and calcined in the furnace. The formation of silver nanoparticles was observed with UV spectroscope, which showed surface Plasmon peaks in the range between 420 and 460 nm, as displayed in Figure 1. The product was characterized by SEM in Figure 2. The SEM image shows that the particle size is about 120 nm.
General procedure for the synthesis of Benzoxazole
A mixture of aldehydes (1 mmol), o-Aminophenol (1 mmol), and zinc nanosulfide (0.003 g) was heated at 70 °C for the appropriate time in solvent-ethanol. The reaction improvement was monitored by TLC (n-hexane: ethyl acetate 1:2). After the completion of the reaction, the mixture was washed with cold ethanol and the crude product was crystallized by ethanol to obtain pure benzoxazole derivatives in 80-96% yield.
Selected data of benzoxazole derivatives
2-(4-nitrophenyl)benzo[d]oxazole
m.p 156-158 °C, IR (KBr, ʋmax cm-1): 1635, 1448, 1420, and 1550 cm-1. 1H-NMR (400 MHz, DMSO): δ 7.5-8.70 (m, 9H aromatic). 13C-NMR (100 MHz, DMSO): δ 110.2, 119.1, 121.3, 122.6, 123.8, 128.5, 132.3, 141.7, 148.0, 150.7, 162.6, and 173.2.
4-(benzo[d]oxazole-2-yl)-N methylbenzenamic
m.p 79-81 °C, IR (KBr, ʋmax cm-1): 3371, 1749, 1542, 1500, and 1150 cm-1. 1H-NMR (400 MHz, DMSO): δ 2.60 (s, 3H, CH3) 4.0 (brs, 1H, NH) 6.44-7.20 (m, 9H aromatic). 13C-NMR (100 MHz, DMSO): δ 29.7 (CH3), 112.2, 118.1, 120.3, 122.6, 123.7, 124.3, 128.5. 141.6, 150.8, 162.8, 169.2, and 173.1.
2-(benzo[d]oxazole-2-yl)-6-methoxyphenol
m.p 192-194 °C, IR (KBr, ʋmax cm-1): 3347, 1550, 1679, and 1538 cm-1. 1H-NMR (400 MHz, DMSO): δ 3.73 (s, 3H OCH3), 5.0 (brs, 1H OH), and 6.43-7.36 (m, 8H aromatic). 13C-NMR (100 MHz, DMSO): δ 55.2 (OCH3), 111.2, 113.1, 115.9, 117.5, 120.1, 134.3, 137.7, 141.2, 150.4, 162.0, 163.1, 168.3, and 172.1.
4-(benzo[d]oxazole-2-yl) phenol
Oil, IR (KBr, ʋmax cm-1): 3343, 1620, 1635, and 1623 cm-1. 1H-NMR (400 MHz, DMSO): δ 5 (brs, 1H OH), and 6.69-7.31 (m, 9H aromatic). 13C-NMR (100 MHz, DMSO): δ 110.4, 112.2, 114.1, 115.9, 116.5, 118.1, 119.5, 128.2, 133.9, 158.4, 162.1, and 168.9.
2-(3, 4-dimethoxyphenyl)-1H-benzo[d]oxazole
Oil, IR (KBr, ʋmax cm-1): 1540, 1630, and 1672, 1683. 1H-NMR (400 MHz, DMSO): δ 3.73 (d, 6H OCH3), 6.88-7.36 (m, 8H aromatic). 13C-NMR (100 MHz, DMSO): δ 56.3 (2OCH3), 111.8, 112.2, 136.5, 139.1, 141.3, 148.2, 149.1, 150.5, 158.2, 159.3, 163.4, 164.1, and 171.2.
Results and Discussion
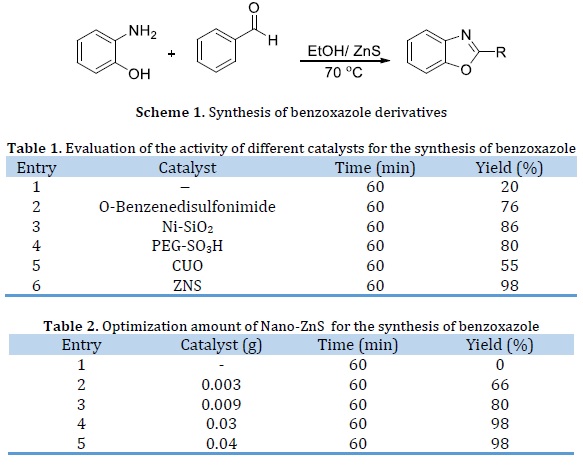
To find the best reaction conditions, the reaction of aldehydes (1 mmol), o-aminophenol (1 mmol) was performed under various conditions and different quantities of nano-sulphid zinc (Scheme 1).
To establish better catalytic activity of nano-sulphid zinc, the reaction was compared using other catalysts at 70 °C and for 10 min under solvent ethanol. The results are listed in Table 1. The problems in the reported protocols such as prolonged reaction time and poor yields prompted us to develop a new rapid method offering excellent yields using a solid-phase basic green catalyst for the benzoxazole synthesis.
To determine the optimum quantity of nano-sulphid zinc, the reaction of aldehydes, o-aminophenol was carried out at 70 °C and for 10 min under solvent ethanol using different quantities of nano-sulphid zinc (Table 2). Nano-ZNS of 0.003 g gave an excellent yield in 60 min (Table 2, entry 3).
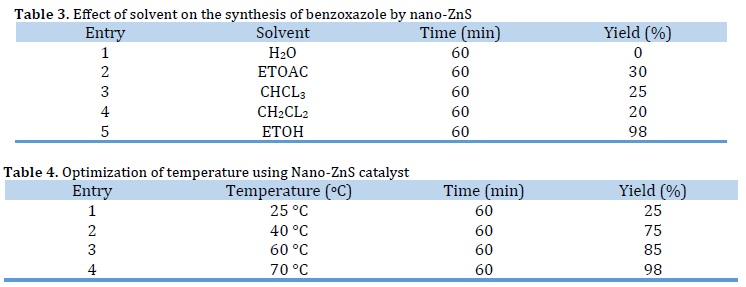
The above reaction was further examined in various solvents (Table 3). The results indicated that different solvents affected the reaction efficiency. Most of these solvents required a longer time and gave moderate yields, and the best results were obtained under solvent ethanol (Table 3, entry 5).
To optimize the temperature in the mentioned reaction, we have carried out a model study with aldehydes, o-Aminophenol using 0.003 g of catalyst at various temperatures (Table 4, entry 4). Table 4 clearly demonstrates at 70 °C is an effective temperature in terms of reaction time and yield.
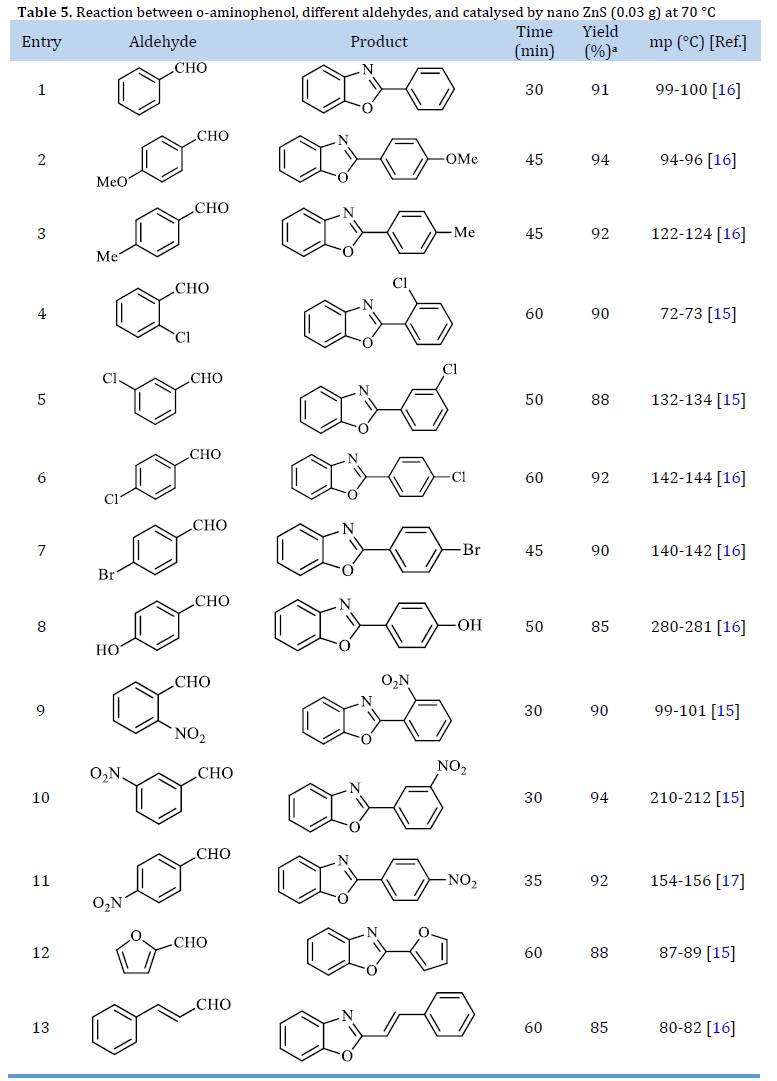
The generality of these conditions to the conversion of various aromatic aldehydes with electron-donating and withdrawing groups to their corresponding benzoxazole was evaluated (See Scheme 1, Table 5). The results revealed that all of the studied aryl aldehydes were completely converted into their respective 2-arylbenzoxazoles with high isolated yields in short reaction times. It is noteworthy that reaction with aldehydes containing electron-withdrawing groups such as nitrobenzaldehydes was accomplished in shorter time than those with electron-donating groups such as 4-methylbenzaldehyde and 4-methoxybenzaldehyde.
Conclusion
In conclusion, nanosulphide zinc simple work-up was developed as inexpensive and gives excellent yields for the synthesis of biologically active o-aminophenol and various aldehyde compounds at 70 °C and ethanol solvent. The present method has many advantages over the previously reported procedures in terms of reduced reaction timings, higher yield, simplicity in operation, and work-up, avoid the application of dangerous acids or bases, environmentally friendliness, simple recovery of nanosulphide zinc and reuse of the catalyst several times without a remarkable decrease in its activity.
Acknowledgements
The Research Council of Payam-e-Noor University is gratefully acknowledged for the financial support for this work.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Orcid
Fatemeh Hakimi
https://orcid.org/0000-0002-4580-4139
How to cite this manuscript: Fatemeh Hakimi*, Elham Golrasan. Zinc sulfide (ZnS) nanoparticles: An effective catalyst for synthesis of benzoxazole derivatives. Asian Journal of Green Chemistry, 7(1) 2023, 47-53. DOI: 10.22034/ajgc.2023.1.6